-
植物经常暴露于各种环境(如干旱、盐碱、重金属等)胁迫中,严重影响了它们的酶和非酶成分,还对它们的相关基因构成威胁,且减缓了同化系统建成速度,影响生长进程。活性氧(ROS)在调节各种生物现象中起着重要的作用,包括激活细胞因子信号转导通路以及由此引起的基因表达。持续暴露于ROS中会引起植物的氧化胁迫,影响细胞内氧化还原平衡。非生物胁迫可导致细胞ROS浓度增加,随后转化为H2O2,它能够跨膜自由扩散,当其在细胞中的浓度积累到一定程度时,就会造成细胞损伤。同样,H2O2也可作为信号分子参与到胁迫信号转导途径中[1]。此外,活性氧不足可以引起细胞氧化胁迫,影响基因组的稳定性[2],因此氧化胁迫是细胞损伤的重要原因。蒋景龙等[3]施加不同浓度H2O2处理7 d苗龄的山黧豆幼苗24 h,分析山黧豆根系受氧化胁迫的程度与抗氧化系统的响应机制,结果表明,H2O2的积累与其受氧化胁迫程度呈正相关,且低浓度处理可以提高山黧豆抗氧化性能。Wan等[4]通过对12 d苗龄的水稻进行不同梯度H2O2处理6 h,分析叶片生理生化响应,并结合蛋白质组学揭示了水稻叶片生理特征变化与其胁迫响应蛋白之间的关系。
脱落酸(ABA)在植物非生物胁迫中起关键作用,它是激发植物应对不利环境条件的重要信号[5-6],并且能够协同调节胁迫反应中多种生理功能,包括气孔闭合,积累渗透调节物质和诱导胁迫相关蛋白的合成,如热休克蛋白,ROS清除剂等。然而,虽然许多非生物胁迫诱导基因受ABA控制,但有一些不是,这表明ABA依赖和非依赖性途径共同参与胁迫信号的传导[7-8]。ABA也是长距离信号分子,当环境条件发生变化时,它能够从成熟叶片持续地传输到发育叶片[9]。胁迫引起H2O2含量增加,造成氧化胁迫,而施加外源ABA,通过其信号转导,诱导相关基因表达,从而提高植物抗性[10-11]。Desikan等[12]研究发现,施加外源ABA有利于氧化胁迫下植物的适应生长,增加抗氧化酶的活性并诱导ROS清除系统等相关基因表达。王允等[13]研究表明,ABA可通过提高姜叶片ROS抗氧化系统的酶和非酶成分以抵御干旱引起的氧化胁迫。另外,研究显示外源物的调控效果与植物种类以及施用的方法、时间和浓度关系紧密[14, 15]。侧柏(Platycladus orientalis (Linn.) Franca)资源丰富,是生态环境修复的主要造林树种,具有一定的耐寒、耐旱、抗盐碱等特性,广泛分布于我国各地区,是我国特色树种,目前关于外源ABA与侧柏氧化胁迫交互作用的研究尚无报道。本试验研究了氧化胁迫下侧柏活性氧代谢以及施加外源ABA对其产生的作用,以期从生理生化和分子方面探讨侧柏的抗氧化胁迫和ABA的调控机制,为其更好地推广利用提供理论依据。
HTML
-
试验是在预备试验的基础上,于2016年在中国林业科学研究院温室大棚内进行。以侧柏幼苗为试材,其种子采集于中国林业科学研究院院内,经浸种处理后,于2016年1月播种于塑料穴盆中,待生长至4月下旬,选择生长一致的植株,将其冲洗干净,转移至含有1/4 Hoagland溶液(pH 6.0)容器(10 L,60株/容器)中培育两周,待幼苗恢复正常生长,对侧柏进行试验处理。根据前期预实验结果,选择100 mmol·L-1 H2O2为胁迫处理浓度。试验处理具体如下:CK(1/4 Hoagland溶液)、CK+ABA0.5(0.5 μmol·L-1 ABA+1/4 Hoagland溶液)、CK+ABA200(200 μmol·L-1 ABA+1/4 Hoagland溶液)、H2O2(100 mmol·L-1 H2O2+1/4 Hoagland溶液)、H2O2+ABA0.5(0.5 μmol·L-1 ABA+100 mmol·L-1 H2O2+1/4 Hoagland溶液)、H2O2+ABA 200(200 μmol·L-1 ABA+100 mmol·L-1 H2O2+1/4 Hoagland溶液)。以上实验每处理3个生物学重复,每个生物学重复20株。所有植株处理6和48 h后取样,且进行液氮快速冷冻并存储在-80℃,为后续RNA提取和生理指标测定分析做准备。
-
H2O2含量测定采用四氯化钛沉淀法[16];MDA含量测定采用硫代巴比妥酸显色法[17];SOD活性测定采用氮蓝四唑(NBT)法[18];POD活性测定方法参照Cakmak和Marschner [19];CAT活性测定方法参照Jablonski和Anderson [20]方法;GSH含量测定方法参照Anderson [21];Proline含量测定采用茚三酮比色法[22];可溶性蛋白测定采用考马斯亮蓝法[23]。
-
采用植物RNA提取试剂盒(Tiandz)提取侧柏叶片的总RNA,并使用PrimeScriptTM RT reagent Kit(Takara)试剂盒反转录合成cDNA。根据侧柏转录组的注释Unigene,采用Primer Premier 3.0软件进行侧柏活性氧代谢相关基因定量引物设计,引物扩增效率均在95%~105%,PCR扩增产物均在100~150 bp,引物序列见表 1,αTUB为侧柏内参基因[24],相对表达量使用2-ΔΔCT方法[25],3个生物学重复。实时荧光定量PCR(qRT-PCR)测定在Roche LightCycler®480进行,使用SYBR®Premix Ex TaqTM II (Takara)试剂盒。PCR反应体系(20 μL)含有10 μL SYBR® primer Ex Taq(2×),每条引物0.8 μL(10 μmol·L-1),cDNA稀释13倍,取2.0 μL和6.4 μL蒸馏水。qRT-PCR反应程序为:95℃预变性10 s;95℃变性15 s,60℃退火30 s,40个循环。反应结束后对扩增产物荧光值变化和熔解曲线进行分析。
基因名称Gene name 引物Primer sequence (5'→3') Cu-Zn超氧化物歧化酶
(Cu/Zn-SOD) Superoxide
dismutase, Cu-Zn familyTTGAGGGCGTTGTGAGTCTC (Forward)
ACCTGTTGACATGCACCCAT (Reverse)过氧化氢酶(CAT)
CatalaseTTGTGAAACGTTGGGTGGGA (Forward)
CTTCTGGCCGAGGGATTTGT (Reverse)抗坏血酸盐过氧化物酶
(APX)
L-ascorbate peroxidaseGGGCTAACAGTGGCTTGGAT (Forward)
ACCTCAACAGCCACAACTCC (Reverse)谷胱甘肽还原酶(GR)
Glutathione reductaseTTGGAGCAGTGGGAGTTGAC (Forward)
GTTACATCACCGACTGCCCA (Reverse)单脱氢抗坏血酸还原酶
(MDAR)Monodehydroascorbate
reductaseTGGGGAGCTTGCCATCATTT (Forward)
TGGAAACCTGGTAGTCGTGC (Reverse)谷胱甘肽S-转移酶(GST)
Glutathionine S-transferaseTTTTGGCCTTGCAACCCTTT (Forward)
GACAAGTCCCCTTTTCCCCA (Reverse)Table 1. The primers of genes related to reactive oxygen metabolism used for qRT-PCR
-
利用Microsoft Excel 2013软件进行数据处理及作图,所有统计分析采用SPSS19.0软件,采用One-Way ANOVA进行比较。各处理之间的显著性差异检验水平为P < 0.05。
1.1. 材料及处理
1.2. 生理指标测定
1.3. 实时荧光定量qRT-PCR检测活性氧相关基因的表达量
1.4. 数据处理
-
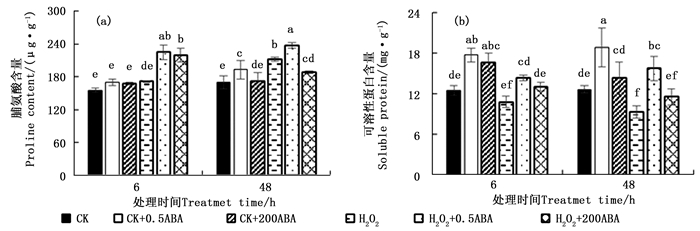
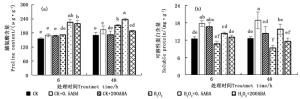
图 1a显示,正常条件下侧柏外施0.5和200 μmol·L-1浓度的ABA,其叶片H2O2含量于6 h分别较对照CK降低27.73 %和2.91 %,处理48 h,分别降低22.16 %和6.74 %;侧柏幼苗响应100 mmol·L-1 H2O2胁迫,其叶片H2O2含量于6和48 h分别较对照CK显著提高22.85 %和43.78 %,且在0~48 h内,随着胁迫时间延长,其含量逐步增加。施加不同浓度ABA对叶片H2O2积累产生不同的效应。H2O2+0.5ABA处理于6 h降低胁迫叶片H2O2水平的13.61 %,而H2O2+200ABA则是提高了4.68 %且与H2O2处理差异显著(P < 0.05),处理至48 h,H2O2+0.5ABA和H2O2+200ABA分别降低20.81 %和12.37 %。由此可知,相较于施加高浓度200 μmol·L-1 ABA,H2O2胁迫外施低浓度0.5 μmol·L-1 ABA更有利于降低H2O2的快速生成。
MDA含量反映胁迫引起的氧化胁迫程度。正常条件下侧柏外施0.5和200 μmol·L-1 ABA处理6和48 h,其叶片MDA含量较对照CK均显著降低(P < 0.05);侧柏小苗H2O2胁迫6和48 h,叶片MDA含量相较于CK分别提高了30.41 %和69.84 %(图 1b),6和48 h处理间叶片MDA含量差异显著(P < 0.05)。H2O2+0.5ABA和H2O2+200ABA处理侧柏幼苗6 h,前者显著降低了MDA含量,幅度为H2O2胁迫的6.02 %,而后者与H2O2处理差异不显著(P>0.05)。随着处理时间延长至48 h,H2O2+0.5ABA和H2O2+200ABA处理均能显著降低胁迫侧柏叶片MDA含量,前者的缓解效果明显高于后者,降幅分别为H2O2胁迫的39.51 %和14.34 %(P < 0.05)(图 1b)。
-
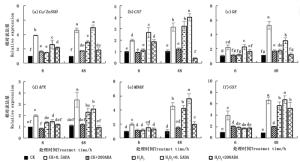
通过测定侧柏幼苗叶片SOD、POD和CAT活性,从而评估ABA对侧柏幼苗的抗氧化酶活性的影响,结果见图 2a~c。100 mmol·L-1 H2O2胁迫处理6 h提高了SOD活性,相较于CK提高10.01 %,但差异不显著(P>0.05);之后这种上升趋势持续到48 h,显著提高了36.80 %,由此可知,侧柏在0~48 h胁迫过程中,其SOD活性是逐步升高的。正常条件下,0.5和200 μmol·L-1 ABA处理侧柏6和48 h均可显著提高SOD活性;而在H2O2胁迫条件下施加不同浓度ABA处理48 h,侧柏叶片SOD活性产生不同的响应。H2O2+0.5ABA处理显著提高了胁迫叶片的SOD活性,幅度为12.30 %,而H2O2+200ABA处理相较于H2O2胁迫叶片降低了28.57 %。100 mmol·L-1 H2O2处理6和48 h导致侧柏叶片POD活性较对照CK下降,且6 h时两者之间差异显著(P < 0.05)。正常条件外施ABA对POD活性的影响与SOD活性类似。H2O2+0.5ABA和H2O2+200ABA处理48 h均显著增加了胁迫叶片的POD活性(P < 0.05),但两者之间差异显著,低浓度(0.5 μmol·L-1)ABA提高了97.49 %,而高浓度(200 μmol·L-1)则增加了25.60 %。CAT活性在100 mmol·L-1 H2O2处理6和48 h后分别较对照CK上升了17.04 %和30.78 %,H2O2+0.5ABA处理较H2O2+200ABA能明显提高叶片CAT活性,6和48 h后分别较对照CK上升了29.40 %和8.80 %。通过以上分析说明,低浓度0.5 μmol·L-1 ABA明显提高胁迫条件下侧柏叶片抗氧化酶活性,更有利于维持叶片活性氧产生和清除之间的动态平衡。

Figure 2. Effects of ABA on superoxide (SOD), peroxidase (POD) and catalase (CAT) activities and GSH contents of P. orientalis under H2O2 stress
图 2d显示,侧柏在正常条件下外施0.5和200 μmol·L-1 ABA,其GSH含量于6 h分别较对照CK显著增加了16.10 % 和9.59 %,之后这种趋势继续,至48 h,分别显著提高26.67 %和16.05 %;侧柏叶片GSH含量经单一100 mmol·L-1 H2O2处理后增加,分别较对照CK提高了46.94 %和35.27 %,且6 h高于48 h,随时间的延长有降低的趋势。而外施0.5和200 μmol·L-1 ABA处理48 h在一定程度上致使H2O2胁迫的侧柏叶片GSH含量产生不同响应,H2O2+0.5ABA处理进一步提高了胁迫叶片GSH含量,H2O2+200ABA处理相较于H2O2胁迫则抑制了GSH的合成。
-
H2O2胁迫下侧柏幼苗外施不同浓度ABA的脯氨酸含量变化趋势见图 3a。正常条件下外施不同浓度ABA处理6 h后,此时脯氨酸含量与对照CK之间差异不显著;48 h后,低浓度0.5 μmol·L-1显著提高了脯氨酸含量(P < 0.05)。单一H2O2处理0~48 h,脯氨酸含量随着时间延长而逐步增加;48 h时,H2O2胁迫与对照CK处理之间差异显著,提高了24.66 %。H2O2+0.5ABA处理48 h提高胁迫叶片脯氨酸含量12.01 %,H2O2+200ABA处理则降低了10.95 %,两者均与100 mmol·L-1 H2O2胁迫处理间差异显著(P < 0.05)。
图 3b显示,侧柏叶片100 mmol·L-1 H2O2处理6 h,胁迫叶片可溶性蛋白含量较对照CK差异不显著(P>0.05);处理48 h,可溶性蛋白含量较对照CK显著降低了26.53 %;可溶性蛋白含量随着胁迫时间(0~48 h)延长逐步下降,H2O2抑制了可溶性蛋白的合成,加速其水解,导致含量下降。正常条件外施0.5和200 μmol·L-1 ABA 6 h均可显著提高侧柏叶片的可溶性蛋白含量,48 h后,200 μmol·L-1 ABA处理的叶片可溶性蛋白含量有降低的趋势,与对照CK差异不显著(P>0.05)。0.5和200 μmol·L-1 ABA处理6和48 h均能提高胁迫叶片可溶性蛋白含量,48 h后前者显著性提高了69.48 %,后者与H2O2胁迫处理间差异不显著(P>0.05)。
-
图 4显示,正常和100 mmol·L-1 H2O2胁迫下外施0.5和200 μmol·L-1的ABA致使侧柏幼苗叶片活性氧代谢相关基因Cu/Zn-SOD、CAT、GR、APX、MDAR和GST表达水平产生变化。正常条件下0.5 μmol·L-1 ABA处理的侧柏叶片中Cu/Zn-SOD、CAT、GR、APX、MDAR和GST表达水平相较于200 μmol·L-1 ABA处理有显著性提高(P < 0.05),于6 h分别较对照CK显著增加了2.87、0.98、1.15、1.00、0.98和2.96倍;处理48 h,分别提高3.82、1.98、3.84、2.03、3.68和5.94倍;侧柏幼苗响应100 mmol·L-1 H2O2胁迫,于48 h其叶片活性氧代谢相关基因表达水平较对照CK均有显著性提高,且在0~48 h内,随着胁迫时间延长,活性氧代谢相关基因表达水平逐步增加。此外,由图 4可知,相较于200 μmol·L-1 ABA,0.5μmol·L-1 ABA处理6和48 h更有利于提高H2O2胁迫条件下侧柏叶片活性氧代谢相关基因Cu/Zn-SOD、CAT、GR、APX、MDAR和GST的表达量。其中6 h处理分别提高了0.79、1.34、0.98、0.07、0.30和-0.10倍;48 h处理提高了0.69、0.24、0.83、0.11、0.32和0.18倍,且随着H2O2胁迫时间(0~48 h)延长,这六个活性氧代谢相关基因表达水平显著提高(P < 0.05)。
2.1. 外源ABA对侧柏叶片H2O2和MDA含量的影响
2.2. 外源ABA对侧柏叶片抗氧化物酶和GSH含量的影响
2.3. 外源ABA对侧柏叶片渗透调节物质的影响
2.4. 外源ABA对侧柏叶片活性氧代谢相关基因表达的影响
-
许多植物已经发展了复杂的胁迫响应机制,改变它们的表型或生理特性来适应环境变化,但持续不利的环境状况均能诱使植物连续地产生ROS,使得ROS的产生和清除之间的平衡关系被打破,从而引起了植物的氧化胁迫。高盐诱导氧化胁迫引起杨树[26]和水稻[27]叶片中H2O2增加,膜脂过氧化程度加剧,致使MDA含量增加;镉胁迫下姜叶片中H2O2和MDA增加[28]。外源ABA可以有效地提高活性氧酶促清除系统活性,降低ROS积累和细胞膜脂质过氧化程度,保护膜的完整性,从而缓解由氧化胁迫引起的植物损伤[7, 8]。外施ABA能够明显提高高粱[29]和柑橘[30]的耐盐性以及姜[13]的抗旱性等。然而,所需的ABA浓度因植物种类的不同有很大差异。在本实验中,H2O2胁迫处理6和48 h引起侧柏幼苗叶片H2O2水平显著增加,致使膜质过氧化作用,导致MDA含量明显升高;侧柏叶片中H2O2和MDA含量随着胁迫时间(0~48 h)的延长而逐步增加;相较于200 μmol·L-1 ABA,H2O2胁迫下侧柏幼苗外施0.5 μmol·L-1 ABA更有利于降低H2O2和MDA的积累(图 1)。
-
植物对短期氧化胁迫具有一定的适应性,短时间胁迫可诱导其抗氧化酶活性及相关基因表达上调。如豌豆[31]和姜[13]的SOD、POD和CAT活性;水稻叶片Cu/ZnSOD、MnSOD、APX和CAT基因[32]、水稻OsAPX8[33]、穇子EcMDAR[34]、芦苇PhaGRC[35]。盐诱导氧化胁迫以及ABA处理均可引起白三叶草Cu/Zn-SOD、CAT、GR、APX和MDAR基因转录水平上调[36]。本研究中,侧柏叶片中SOD和CAT活性经H2O2胁迫处理48 h后显著增加(图 2),表明侧柏幼苗对48 h短期胁迫具有一定的适应性,可能和ROS水平上升诱导活性氧清除酶基因的表达水平有关[37]。而POD活性在6 h胁迫处理后相较于对照CK显著降低,随着胁迫时间延长至48 h,其活性缓慢上升,与对照CK处理间差异不显著,;100 mmol·L-1 H2O2处理48 h上调侧柏幼苗叶片Cu/Zn-SOD、CAT、GR、APX、MDAR和GST基因的表达水平(图 4)。目前研究表明,ABA作为植物胁迫响应中的重要信使,能够通过提高ROS清除酶活性以及相关基因的表达来提高植物的抗氧化胁迫能力[38-41],还能通过增加GSH含量来增强植物的抗氧化胁迫能力[13]。如黄麻干旱胁迫45 d,叶片中的SOD和CAT活性被抑制,外施ABA能够降低H2O2含量,提高植株活性氧清除酶SOD和CAT活性,缓解氧化胁迫[42]。编码ROS清除酶基因的过量表达,如SOD[43]、CAT[44]、APX[45]、MDAR[46]、GR[47]和GPX[48],能够通过降低氧化损伤提高植物对不同非生物胁迫抗逆性。本研究中,正常和100 mmol·L-1 H2O2侧柏幼苗外施0.5 μmol·L-1 ABA均能进一步增加抗氧化酶SOD、POD和CAT活性(图 2);相较于200 μmol·L-1 ABA,外施0.5 μmol·L-1 ABA更能诱导这些基因的表达水平显著提高。胁迫条件下,随0.5 μmol·L-1 ABA处理时间(0~48 h)的延长,Cu/Zn-SOD、CAT、GR、APX、MDAR和GST基因的表达水平呈升高趋势,较H2O2胁迫均有不同程度地上升(图 4)。
-
脯氨酸是在环境胁迫条件下积累在许多生物体中的一种重要的有机分子[49]。一些研究人员认为脯氨酸积累可能与胁迫程度[50, 51]或渗透调节[52]有关;相反地,也有一些专家则认为这是胁迫损伤的症状而不是抗性指标[53]。本研究中,H2O2胁迫诱导侧柏叶片脯氨酸含量增加(图 3a),处理48 h的脯氨酸含量高于6 h,随着胁迫时间的延长而增加,可能与其细胞渗透调节机制有关[54],这与燕麦脯氨酸的研究趋势一致[55]。100 mmol·L-1 H2O2胁迫下侧柏幼苗外施0.5 μmol·L-1 ABA处理48 h相较于200 μmol·L-1,脯氨酸水平显著性提高(图 3a),可能是因为低浓度0.5 μmol·L-1 ABA更利于促进脯氨酸生物合成中相关酶的活性,延缓其降解,致使侧柏叶片脯氨酸的积累从而增强植株的渗透调节能力。这一情况同样出现在豆芽的研究中,即Khadri等[56]指出低浓度的ABA(1 μmol·L-1)增加了普通豆芽中的脯氨酸积累,提高了对氧化胁迫的耐受性。另外,在研究中,H2O2处理0~48 h,侧柏叶片可溶性蛋白含量逐步下降;施加0.5 μmol·L-1 ABA后明显提高侧柏叶片中可溶性蛋白含量,200 μmol·L-1 ABA与胁迫处理差异不显著(图 3b),表明0.5 μmol·L-1 ABA浓度更利于促进H2O2胁迫下侧柏蛋白合成。
3.1. 高、低浓度ABA对侧柏叶片活性氧和膜脂过氧化的影响
3.2. 高、低浓度ABA对侧柏叶片抗氧化酶活性及其相关基因表达的影响
3.3. 高、低浓度ABA对侧柏叶片渗透调节物质的影响
-
H2O2胁迫引起侧柏叶片H2O2水平显著增加,致使MDA含量明显升高;而侧柏叶片中SOD和CAT活性经H2O2胁迫处理48 h后显著增加,表明侧柏幼苗对短期胁迫具有一定的适应性,短期胁迫诱导抗氧化酶相关基因表达上调。正常和H2O2胁迫条件下的侧柏幼苗,相较于200 μmol·L-1 ABA,外施低浓度0.5 μmol·L-1 ABA均有利于维持叶片较高的抗氧化酶(SOD、POD和CAT)活性和抗氧化物GSH含量,降低H2O2和MDA积累,增强渗透调节能力,同时更能诱导抗氧化酶相关基因表达水平显著提高,表明外施ABA有利于提高抗氧化系统的防御能力,减弱幼苗的氧化胁迫和膜脂过氧化水平,保护细胞膜结构的完整,从而有效地缓解逆境胁迫对植物造成的氧化伤害。





 DownLoad:
DownLoad: