-
在很多交配系统中,物种选择配偶时必须定位和识别不同质量的个体。准确、高效的识别和评估配偶需要不同物种个体根据其交配环境利用合适的交配信号和感官通道。此外,动物可能进化出通过多种感官信号来识别配偶以应对波动的和多样化环境[1-2]。昆虫识别配偶的关键信号可能通过视觉[3-5]、嗅觉[6-8]、听觉[9-11]和触觉信号等途径来传达[12]。视觉信号可能涉及色彩、翅大小、运动模式和形状等[13-14]。嗅觉信号是由雄性或雌性释放的,以吸引配偶或同种个体,在近距离或远距离发挥作用的挥发物[15-17]。
蝶类的两性交流涉及在配偶识别和不同选择阶段使用多个信号,从而构成复杂的场景。蝶类求偶一般是雄蝶追求雌蝶,雄蝶主动而雌蝶被动。求偶中的蝶类发现同类后,通常会上前追逐(即开始求偶),雄蝶的该行为通常都是靠视觉而非嗅觉或其他因素引起[18-19];而在追逐的过程中通过视觉、嗅觉或触觉等信息辨别同类及异性配偶[20]。蝴蝶在广泛的可见光谱范围内有良好的色彩辨别能力[21],很多蝶类的翅除了有多彩的颜色外,还有反射紫外线的能力[20, 22]。菜粉蝶(Pieris rapae rapaeL.)雌雄蝶在翅颜色上有差异,雄蝶靠视觉区别雌雄两性[23]。雄性灰蝶(Zizeeria maha argia Ménétriès)不能仅依靠视觉来辨别异性,因为雌雄蝶的翅下表面反射模式几乎相似,雄蝶通过嗅觉辨别雌雄蝶[19, 24]。在蝶类中,雄蝶在求偶过程中的两性识别阶段对视觉和嗅觉等信号的利用有较多报道[23-24],但雌蝶利用何种信号来辨别雌雄两性的相关研究相对较少。
本研究以白带锯蛱蝶为对象探究雌蝶种内识别时对视觉和嗅觉信号的利用。白带锯蛱蝶(Cethosia cyane cyane Drury)(鳞翅目:蛱蝶科)又名泰裙纹蛱蝶、齿缘白带蛱蝶和紫白蝶等,国内分布于云南、广东、广西、四川和海南等,国外分布于中南半岛和印度尼西亚[25]。成虫中型,雌雄异型。幼虫寄主为西番莲科(Passifloraceae)蒴莲属(Adenia)的三开瓢(A.cardiophylla(Mast.) Engl)和滇南蒴莲(A.penangiana)[26]。目前,有关白带锯蛱蝶的研究主要有生物学特性[27-28]、种群生命表[29]、其幼虫和蛹的营养成分[30]、觅食过程中对视觉和嗅觉信号的利用途径[31]、生物地理学及系统分类学[32]等。但未见有关白带锯蛱蝶利用何种信号识别同类及异性的报道。
本研究通过探究视觉和嗅觉信息在白带锯蛱蝶雌蝶的异性识别扮演中的作用,以及探讨:(1)雌蝶是否可仅靠视觉信息识别雌雄两性;(2)雌蝶是否可仅靠嗅觉信息识别雌雄两性;(3)雌蝶是否较偏爱同时有视觉和嗅觉信息的雄蝶。以期为深入了解性二型蝶类两性交流机制提供科学依据。
HTML
-
试验在云南省元江县中国林科院资源昆虫所元江试验站(102°00′ E, 23°36′ N)进行。海拔400 m,年平均气温19~20℃,年平均降水量500~600 mm,属于干热气候。
-
白带锯蛱蝶来自元江试验站人工养殖的种群。幼虫饲喂寄主植物三开瓢,在(27±2)℃,光照条件为13L∶11D,湿度为60%的条件下饲养。行为实验所用成虫均为羽化第3~4天未交配的成虫(雌雄成虫均能够在羽化第2天交配)。
雌雄蝶视觉模型。翅模型:将羽化当天的成虫处死,放置30 d后用镊子将翅小心取下,分别将雌雄翅按照背腹面粘在黑色塑料条上(2.0 cm×0.6 cm),翅处于平展状态,再粘上黑色假触角。打印的彩色纸模型:将制作的翅模型用复印机扫描后,再用喷墨相片纸打印出来,按照翅轮廓剪裁而成。
雌雄蝶嗅觉模型:实验开始前12 h将羽化4 d的雌雄蝶翅背面用双面胶粘牢,翅合拢仅露出腹面颜色,然后用黑色记号笔将雌雄腹面颜色涂成黑色。
-
在4 m×2 m×2 m的网室内分别悬挂白带锯蛱蝶雄蝶和雌蝶的翅模型各2个,2模型间距40 cm,模型距网室顶部15 cm。网室内放入30只羽化3~4 d的未交配雌蝶,每次观察20 min,记录在此期间雌蝶对雌雄模型的接近(雌蝶朝模型飞来,进入以模型为圆心,半径20 cm的区域,不接触模型,时间不少于2 s)和触碰行为(雌蝶触碰到模型),观察时间为雌蝶较活跃和求偶交配较多的15:00—18:00,雌雄模型均重复4次。
为进一步排除气味等信息的影响,用打印的雌雄蝶彩色纸模型代替翅模型,重复以上实验。所用成虫为30只羽化3~4 d的未交配雌蝶,每次观察20 min。雌雄模型均重复4次。访问次数为接触和触碰模型次数之和。
-
在4 m×2 m×2 m的网室内分别悬挂雄蝶和雌蝶嗅觉模型(活蝴蝶,翅处于合拢状态)各2个,2模型间距40 cm,模型距网室顶部15 cm。网室内放入30只羽化3~4 d的未交配雌蝶,每次观察20 min,记录在此期间雌蝶对模型的接近和触碰行为,观察时间为15:00—18:00。雌雄模型均重复4次。
-
在4 m×2 m×2 m的网室内分别悬挂正常雄蝶和雌蝶(均为羽化4天的活蝴蝶,用线轻轻系在成虫腹部将其悬挂,此时飞行行为与自由飞行相比无明显差异)各2个,2只蝴蝶间距40 cm,距网室顶部15 cm。网室内放入30只羽化3~4 d的未交配雌蝶,每次观察20 min,记录在此期间雌蝶对正常蝴蝶的接近和触碰行为,观察时间为15:00—18:00。雌雄蝶均重复4次。
-
利用Microsoft Excel 2010软件绘图,利用SPSS 18.0进行数据统计分析,差异性检验采用Wilcoxon秩和检验,计算方法参考文献[33]。
1.1. 试验地点
1.2. 试验材料
1.3. 试验方法
1.3.1. 视觉模型试验
1.3.2. 嗅觉模型试验
1.3.3. 视觉+嗅觉模型试验
1.4. 数据分析
-
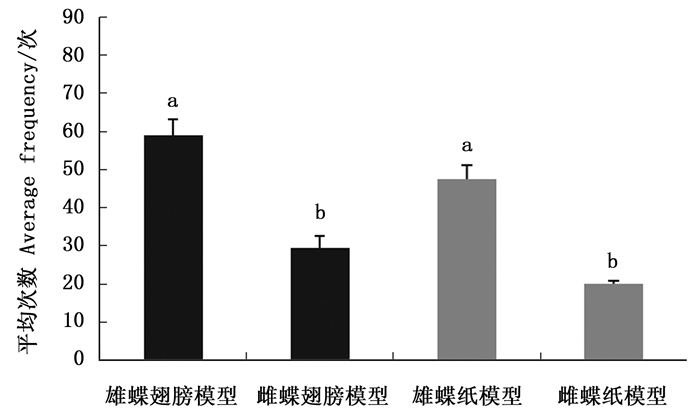
排除白带锯蛱蝶雌雄成虫气味信息后,雌蝶对雄蝶模型(翅和纸模型)访问次数均大于雌模型,对雄蝶和雌蝶翅模型访问次数均大于相应的纸模型(图 1)。雌蝶对雌雄翅模型访问平均次数有显著差异(Z=-2.323,P=0.020);雌蝶对雌雄纸模型访问平均次数有显著差异(Z=-2.323,P=0.020),说明白带锯蛱蝶雌蝶仅靠视觉信息能够辨别雌雄。
-
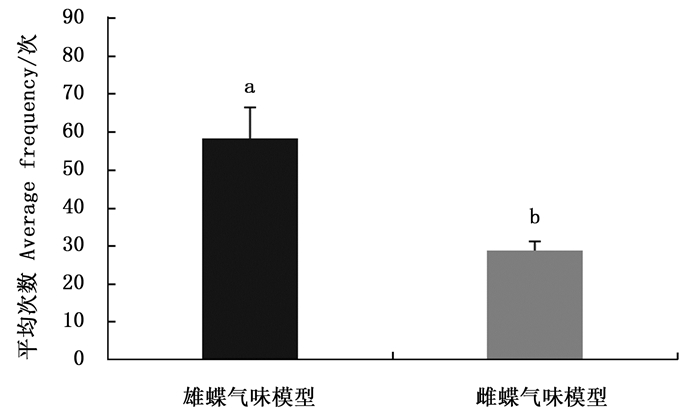
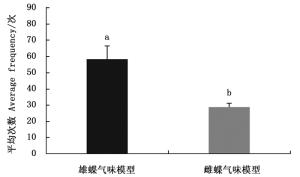
排除白带锯蛱蝶视觉信息仅留下雌雄成虫的气味信息时,雌蝶对雄蝶气味模型的访问平均次数多于雌蝶(图 2)。雌蝶对雌雄嗅觉模型访问平均次数有显著差异(Z=-2.366,P=0.018),说明白带锯雌蝶仅靠嗅觉信息能够辨别雌雄。
-
白带锯蛱蝶雄蝶的视觉和嗅觉信息均存在时,雌蝶对正常雄蝶的访问平均次数多于雄蝶翅和嗅觉模型,对雄蝶翅和嗅觉模型访问平均次数基本无差异(图 3)。雌蝶对正常雄蝶的平均访问次数与雄蝶翅模型(Z=-2.323,P=0.020)和嗅觉模型(Z=-2.309,P=0.021)相比均有显著差异,说明雌蝶识别雄蝶时偏好两种信息的综合作用。
2.1. 白带锯蛱蝶雌蝶识别两性时对视觉信号的利用
2.2. 白带锯蛱蝶雌蝶在识别两性时对嗅觉信号的利用
2.3. 白带锯蛱蝶雌蝶在识别两性时对视觉和嗅觉信号利用权重
-
蝴蝶羽化后进入成虫期,首要任务便是完成交配产卵等生殖过程,交配时面临的主要问题是雌雄蝶如何识别同种异性配偶,以防止错误交配。蝴蝶在求偶过程中可通过视觉、嗅觉、触觉等多种方式识别异性配偶。本研究发现白带锯蛱蝶雌蝶可同时利用视觉和嗅觉信息识别雌雄两性。在昆虫中,同时利用多种信号识别配偶较常见,如视觉和嗅觉信息在雌性丛林斜眼褐蝶(Bicyclus anynanaButler)选择配偶时扮演同等重要的作用[34]。雄性猎狼蛛(Schizocosa crassipes Walckenaer)的视觉和振动信号都足以导致成功交配,而雌性交配时偏好同时展现两种信号的雄性[35]。
白带锯蛱蝶雌蝶利用视觉或嗅觉信息均能辨别两性,这种双保障机制在雌蝶交配中有重要作用。该蝶成虫寿命较短(一般小于10 d),因此, 准确识别异性配偶完成交配至关重要。仅通过视觉信号常常无法准确判断配偶,导致雄蝶追逐错误的对象,而嗅觉信号可能受到风和温度的影响而变化[14],同时依靠2种信息准确识别配偶的概率比仅靠一种信息的大。本研究还发现,羽化4 d后的雄蝶翅鳞片经常脱落和破损,改变了翅视觉信息,此时雌蝶识别雄蝶可能更多地依靠嗅觉信息。因此,当一种信号不起作用时,另一种信号还可以替代和补充,以完成交配过程。
白带锯蛱蝶雌蝶对雄蝶和雌蝶翅模型访问次数均大于相应的纸模型(图 1),可能是打印的翅纸模型与真实的翅差异造成的。纸模型与真实翅相比,不仅亮度减少,还缺少紫外反射能力(未发表数据),这些都可能减少纸模型对雌蝶的吸引力。
在动物交流中同时使用多个信号是常见的,研究者提出了几种假说以解释在求偶示爱中不同信号的进化[1, 36]。多通路的信号可能会提供给接收者有关配偶质量的信息(多重信息假说);或者提供相同的信息,增加物种评估的准确性和质量(备份假说)[37-38];亦或在物种识别和配偶评估方面均发挥作用(物种识别)[39]。本研究发现,雌蝶利用视觉或嗅觉信息均能识别两性,符合物种识别假说,且视觉和嗅觉所起的作用相同,均是给雌蝶辨别雌雄提供依据,并增加雌蝶辨别两性的准确度,因此,也符合备份假说。
白带锯蛱蝶雌蝶识别两性所利用的视觉信号包括翅大小、颜色和紫外反射能力,具体利用哪种信号有待验证。雌蝶识别两性所利用的嗅觉信号主要是身体挥发物,雌雄身体挥发性化合物已测出(未发表数据),从组成和含量上看雌雄差异较小,仅有雪松醇cedrol为雌蝶特有。行为实验表明,雪松醇在雄蝶识别雌蝶过程中发挥作用(未发表数据),那么雪松醇是否为雌蝶识别雌蝶的依据还有待验证,是否还有其他化合物参与雌蝶识别两性的过程仍有待验证。
-
白带锯蛱蝶雌蝶利用视觉或嗅觉信息均能识别雌雄两性,雌蝶在识别两性时较偏爱同时有视觉和嗅觉信息的雄蝶。这种双保障机制在雌蝶交配中有重要作用,同时依靠2种信息准确识别配偶的概率比仅靠一种信息的大,有利于雌蝶在多变的环境中快速识别合适配偶。





 DownLoad:
DownLoad: