-
红树林是生长在沿海潮间带的木本植物群落,是陆海生态过渡带特有的湿地生态系统,在维持海湾河口生态系统的稳定和平衡中起着不可替代的作用[1]。大多数红树植物对寒潮、霜冻等低温天气敏感,低温严重影响红树林幼苗在海岸滩涂地的自然定着和存活[2]。秋茄(Kandelia obovata Sheue et al)作为一种抗寒力强的红树植物[3],在我国分布最广,分布纬度最高,其天然分布北界至福建福鼎(27°20′N),人工引种北界至浙江乐清[4]。2008年历史上罕见的持续低温雨雪冰冻天气对我国南方沿海红树林造成不同程度伤害,秋茄却没受到寒害的伤害。前人对秋茄耐寒特性的研究表明,秋茄生长环境最低月均温为12℃,气温低于5℃时产生寒害[5-6]。杨盛昌等[7]研究了秋茄抗寒力与水分、叶绿素、可溶性蛋白质含量及抗氧化酶活性的相关性;陈鹭真等[5]2008年对我国红树植物寒害受损状况的调查表明,进行抗寒锻炼可提高引种植物的抗寒能力;雍石泉等[8]通过监测秋茄、无瓣海桑(Sonneratia apetala Buch-Ham.)和拉关木(Leguncalaria racemosa Gaertn.f.)幼苗叶片相溶性物质含量及活性氧代谢等生理生化指标发现,秋茄可溶性总糖含量、超氧化物歧化酶(SOD)活性、过氧化物酶(POD)活性均高于无瓣海桑和拉关木;郑春芳等[9]研究了短期夜间低温对植株碳氮代谢相关酶活性的影响,阐述了秋茄幼苗碳氮代谢对夜间短期低温的响应机制。水分利用率(WUE)对环境(干旱、低温等)胁迫响应的研究热点为WUE对干旱胁迫的响应[10-15], 对低温响应的研究甚少[16],而红树植物WUE对低温胁迫响应的研究目前还未见报道。
植物光合作用过程最易受低温胁迫的伤害[17],光合作用是植物生长发育的基础[18-20],WUE是植物能否适应极限环境条件的关键因子[21]。本研究以2月龄秋茄幼苗为材料,研究连续5 d不同低温胁迫对其叶片光合速率(Pn)等光合指标的影响,并阐明其叶片WUE对不同低温的响应机制, 以期探讨秋茄的生存适应对策,为抗寒性红树植物种的选育、引种提供参考。
HTML
-
以秋茄幼苗为试验材料,于2015年5月1日在福建省林业科学研究院潮汐模拟实验室内进行。从漳江口红树林自然保护区采集发育正常的500株秋茄胚轴栽植于10 cm×12 cm×6 cm的营养袋中,袋中装入原生长地潮间带土,培养液用速溶海盐与自来水配制为8‰的人工海水,每天早晚各浇此水1次。
秋茄幼苗培养60 d(7月1日)时,幼苗均长出4片叶子。选取生长正常、大小一致的幼苗300株,移至6个RXZ-280B智能型人工气候箱中的塑料托盘内(50株·箱-1),进行低温胁迫处理。试验采用完全随机区组设计。本试验设计3组处理:常温培养(对照CK)、生存极限低温胁迫(12℃)、寒害低温胁迫(5℃),每组处理100株(2箱),持续胁迫5 d。胁迫期间托盘内8‰的盐水深度保持1 cm左右,并保持设定的处理温度。胁迫期间每组每天取样1次20株,在第1天、第3天、第5天的取样中,分别在各组处理中随机取8株,进行光合参数和叶绿素荧光参数测定,其余12株全部解除胁迫,常温继续培养60 d(至9月1日)后,测定各项指标。
-
用刻度尺测量秋茄胚轴顶端到幼苗顶端的高度。
-
摘取叶片,用扫描仪扫描,用Arc GIS10软件矢量化叶片轮廓后,获取每片叶子的面积和周长,并统计叶片数量。
-
称植株根、茎、叶鲜质量,然后用水洗净,105℃杀青15 min,最后75℃烘干至恒质量,称干质量。
-
测定时间选在晴天上午9:00—11:00,用便携式光合作用测定仪(Li-6400XT)测定光合参数净光合速率(Pn)、胞间CO2浓度(Ci)、气孔导度(Gs)及蒸腾速率(Tr),计算水分利用率(WUF)(WUF=Pn/Tr)。每株测定下面的2片叶子,取平均值。
-
用便携式脉冲调制叶绿素荧光仪(PAM-2500)测定叶绿素荧光参数(与光合作用参数测定同时进行),每株取下面2片叶片,每片叶测定3点。首先夹上暗适应叶夹,叶片暗适应30 min后,获取初始荧光(Fo)、最大荧光(Fm)、可变荧光(Fv=Fm-Fo)及光系统Ⅱ(PSⅡ)最大光能转换速率(Fv/Fm)。
-
用EXCEL表进行数据整理与制图,使用SPSS13.0软件统计分析。
2.1. 苗高、叶面积、叶片数及生物量测定
2.1.1. 苗高测定
2.1.2. 叶面积测定和叶片数量统计
2.1.3. 生物量测定
2.2. 光合参数测定
2.3. 叶绿素荧光参数测定
2.4. 数据分析
-
从表 1可看出:5℃与12℃胁迫均对秋茄幼苗生长产生抑制作用,各指标的生长量均低于CK。5℃持续胁迫2 d,幼苗各指标值均低于胁迫1 d的,胁迫3、4、5 d后,幼苗分别在去除胁迫后第33、23、19天后死亡;12℃持续胁迫5 d,虽然对幼苗各指标的生长产生影响,但随着处理时间的持续,影响程度逐渐降低。这说明5℃胁迫累积超过2 d是秋茄幼苗寒害致死的阈值,而12℃低温环境秋茄幼苗能够适应。
处理 Treatments 胁迫后存活率
Surving rate after stress/%120 d后存活率
Surving rate after 120 days/%叶面积
Leaf areas/cm2叶周长
Leaf perimeter/cm叶片数
Leaf numbers/piece高生长
Height/cm整株鲜质量
Whole plant fresh weight/g温度
Temperature/℃时间
Time/d5 1 100 100 11.86a 13.52ab 4a 12.6e 21.45 2 100 100 12.06a 14.35ab 4a 10.75d 19.76 3 100 0 — — — — — 4 100 0 — — — — — 5 100 0 — — — — — 12 1 100 100 15.48b 14.35ab 4c 12.93d 22.19 2 100 100 9.09a 11.95a 2a 11.33a 19.91 3 100 100 10.54ab 12.92ab 5d 11.35a 20.57 4 100 100 14.88b 14.70bc 2a 11.93b 20.70 5 100 100 13.35ab 15.03bc 3b 12.47c 21.59 CK 100 100 16.73 16.91 9 20.55 26.04 注:表中同列不同小写字母表示不同处理间差异显著性(P<0.05)。
Note: Different letters above show significant difference among treatments(P<0.05).Table 1. Morphological indicators and biomass of Kandelia obovata seedlings in low temperature stress
-
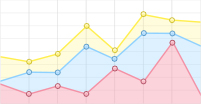
由图 1A、B看出:随着胁迫时间的延长,2个低温胁迫下,秋茄幼苗叶片的净光合速率(Pn)与气孔导度(Gs)均显著下降,但变化趋势不同。5℃胁迫1、3、5 d的Pn分别为CK的35.84%、20.95%、13.68%,Gs分别为CK的21.63%、20.71%、16.12%,Pn与Gs均呈连续下降趋势;12℃胁迫1、3、5 d的Pn分别为CK的26.06%、23.81%、23.99%,Gs分别为CK的13.61%、12.14%、16.41%,Pn与Gs均表现为先降后升。
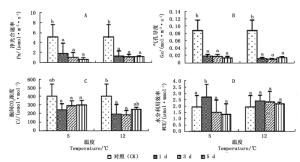
Figure 1. Changes in photosynthetic parameters of Kandelia obovata seedlings under different temperature stresses
图 1C显示:2个低温胁迫下,秋茄幼苗的胞间CO2浓度(Ci)均低于CK。5℃胁迫1、3、5 d,Ci分别为CK的60.64%、72.88%、74.50%,差异不显著,变化趋势与Pn的相反;12℃胁迫下,Ci分别为CK的48.36%、46.17%、62.17%,差异显著。5℃胁迫的Ci比12℃胁迫的高, 这说明,随着胁迫时间的累积,5℃胁迫下秋茄幼苗叶片的光合能力逐渐衰减,而12℃胁迫下光合能力逐渐恢复。
图 1D显示:12℃低温胁迫下,秋茄幼苗叶片的水分利用效率(WUE)差异不显著。5℃胁迫1、3、5 d,WUE分别为CK的140.54%、77.96%、69.95%;12℃胁迫1、3、5 d,WUE分别为CK的123.99%、120.75%、112.70%,均高于CK,但增长率减少。这表明,秋茄幼苗叶片在5℃胁迫1 d和12℃持续胁迫5 d下,具有更高的WUE。
-
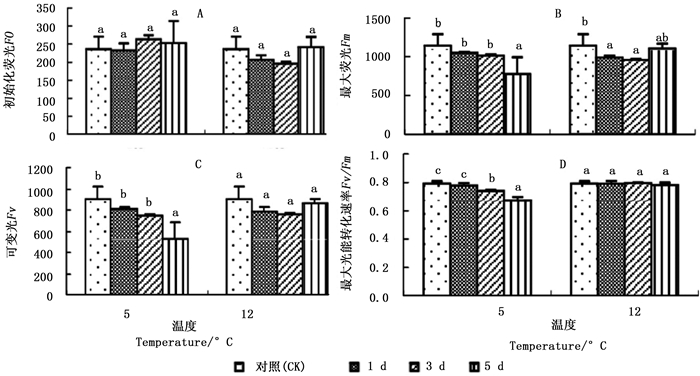
图 2A显示:2个低温胁迫1、3、5 d,秋茄幼苗的初始荧光(Fo)变化率与CK相比均差异不显著。5℃胁迫下的Fo分别为CK的98.30%、111.37%、106.95%;12℃胁迫下的Fo分别为CK的87.17%、82.90、102.10%。
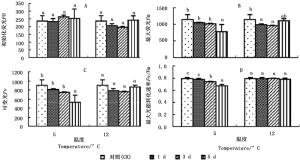
Figure 2. Changes in fluorescence parameters of Kandelia obovata seedlings under different temperature stresses
图 2C显示:2个低温胁迫1、3、5 d,秋茄幼苗的可变荧光(Fv)均低于CK,仅5℃胁迫第5天与CK差异显著。5℃胁迫下的Fv分别为CK的90.19%、81.95%、57.45%,12℃胁迫下的Fv分别为CK的86.36%、83.63%、97.14%。
图 2D显示:5℃胁迫1、3、5 d的最大光能转化速率(Fv/Fm)分别为CK的98.18%、93.24%、84.81%,且胁迫第3、5天与CK差异显著;12℃胁迫1、3、5 d的Fv/Fm分别为CK的99.77%、100.27%、98.62%,差异不显著。5℃胁迫下的Fv/Fm低于12℃胁迫的Fv/Fm,说明5℃下光系统受损程度严重,这与5℃胁迫下的Pn较低有关(图 1A)。
3.1. 秋茄幼苗生长表现
3.2. 秋茄幼苗叶片光合参数变化
3.3. 秋茄幼苗叶片荧光参数变化
-
大多数植物叶片在低温下,Pn与Gs明显下降,导致Pn降低既有气孔限制也有非气孔限制[9, 22-23]。根据Farquhar等[24]的观点,当Pn和Gs下降的同时,Ci上升,则Pn的下降主要受非气孔因素限制,否则为气孔因素限制。本研究发现,5℃与12℃胁迫均使秋茄幼苗叶片的Pn和Gs下降,但Ci变化不同:12℃低温胁迫在第5天出现Gs与Ci同步回升,表明Pn下降的主导因子由非气孔因素限制变为气孔因素限制。这说明秋茄幼苗对于12℃持续胁迫产生了生理适应,这与其生物量回升的变化一致;5℃持续胁迫下,幼苗叶片的Pn和Gs下降,Ci先降低后升高,表明随着胁迫的持续累积,Pn下降由气孔因素限制占主导地位转变为非气孔因素限制占主导地位[25-26]。非气孔限制因素可能是由于胁迫的持续累积影响了PSII的效能,使PSII受损或部分失活[27]。
-
在叶绿素荧光参数中,Fv/Fm不仅反映植物潜在光合能力,同时也反映植物受胁迫程度。任何对PSII效能产生影响的环境胁迫均会导致Fv/Fm降低[28]。本研究中,5℃低温胁迫下,随着胁迫时间的延长,Fv和Fv/Fm均明显下降,而Fo变化微小,这表明用于光合作用的能量减少,转化为叶绿素荧光和热的能量增加,PSⅡ光能转换率下降,潜在活性中心受到损伤,从而对秋茄幼苗叶片光合作用的原初反应产生了抑制作用[29];12℃低温胁迫下,随着胁迫时间的延长,Fv先下降后回升,Fv/Fm变化不明显,表明12℃低温胁迫虽然影响了秋茄的正常光合作用过程,使PSⅡ原初光能转换效率短暂下降,原初反应短暂抑制,但潜在活性中心未受损。
-
水分利用率的大小反映植物进行光合作用的潜能及其逆境适应能力的强弱[7, 15, 18]。图 1D显示:低温胁迫第1天,5℃与12℃胁迫均表现出WUE高于CK,说明在低温前期秋茄幼苗叶片通过增大WUE对逆境产生生理对策,进行自我保护。第3天及第5天,5℃低温胁迫的WUE已显著低于CK,呈连续下降,表明随着5℃胁迫的持续,秋茄幼苗叶肉细胞光合器官受损伤逐渐加重,光合活性逐渐“失活”[30-32],继而出现水分胁迫,导致Pn连续下降(图 1A),细胞中物质和能量代谢失调;12℃胁迫下的WUE持续高于CK,呈现出先增加后稳定的趋势,表明秋茄幼苗叶片能够通过调节WUE,并且提高Ci值,使光合速率保持在一定的水平[21],光合产物逐渐回升。这与前文得出的Pn及生长量变化结论基本一致,前人研究也很好地证实了秋茄幼苗这种生理自我调控的生存对策[33-35]。Passioura根据植物适应策略,将植物分为保守和挥霍两种类型,分别代表较高和较低WUE植物种群,据此分类,秋茄属于保守型植物类型。秋茄的这种遗传特性使秋茄具有较强的抗寒性。
4.1. 秋茄幼苗叶片光合特征
4.2. 秋茄幼苗叶片荧光参数特征
4.3. 水分利用率对寒害的防御机制
-
本研究结果显示:秋茄幼苗在12℃胁迫下,形态指标和生物量减少,5℃胁迫持续2 d是其生存阈值;当低温胁迫未使秋茄幼苗叶片PSII潜在活性中心受损时,Pn下降主要受气孔因子限制;低温胁迫下,秋茄幼苗叶片具有较高的WUE,减缓了光合速率的下降,增强了秋茄的抗寒能力。
浙江乐清湾是秋茄人工引种成功的最北缘。根据中国气象科学数据共享服务网(http://datd.cma.cn)近8年(2011—2018年)的数据,浙江乐清最冷月平均气温为7.4℃、日最低平均气温为5.36℃、日最高气温不超过11℃、日最低气温低至-1.2℃一年中不多于2次。本研究结果显示,5℃胁迫持续2 d是秋茄幼苗的生存极限,12℃胁迫下可存活。由此本研究认为:限制秋茄向高纬度分布的主要环境因子不仅仅是低温,更重要的是极端低温的频率、强度及其持续时间等因子。




 DownLoad:
DownLoad: