-
杨树是我国短周期工业用材林的主栽树种之一。近年来,随着人工林面积的增加及品种单一化,杨树的抗病性显著下降。杨树溃疡病是危害杨树主要枝干病害之一[1],是由葡萄座腔菌属(Botryosphaeria)、腐皮壳菌属(Valsa)和壳梭孢属(Fusicoccum)等病原真菌侵染引起的。在我国,杨树溃疡病主要是由葡萄座腔菌属引起的水泡型溃疡病[2]。水泡型溃疡病在东北、华北、西北等地区大面积流行,给杨树生产造成了极大的损失。化学手段防治杨树溃疡病不仅收效甚微且费用高,还可能污染环境。因此,培育抗病品种已成为当前防治杨树溃疡病的重要途径[3]。
研究表明,将抗病基因转入杨树,能够显著提高杨树的抗病性。例如,转无色花色素还原酶基因(PtrLAR3)的毛白杨对杨盘二孢菌的抗性显著增强[4];过表达WRKY转录因子(PtoWRKY60)基因及过表达拟南芥MYB115基因的转基因毛白杨对溃疡病的抗性明显增强[5-6];转毛白杨WRKY转录因子基因(PtrWRKY18和PtrWRKY35)的毛白杨对病原菌栅锈菌的抗性明显提高[7]。
库安托杨(Populus × euramericana ‘Guariento’)是我国从意大利引进的优良品种,但其抗溃疡病等枝干病害的能力较差。2006年,王建革等[8]将5个外源基因(JERF36、SacB、vgb、BtCry3A、OC-1)转入库安托杨,获得了对多种逆境胁迫均具有较强抗性的转基因株系[9-12]。在5个转入的外源基因中,JERF36是从野生番茄中克隆的ERF (ethylene responsive factor,乙烯应答因子)类转录因子基因[13],在抗病信号传导过程中起重要的作用[14],但其在杨树抗病性中的作用尚未见报道。
本研究对1年生转多基因库安托杨株系(D5-19、D5-20、D5-21)及非转基因对照株系(D5-0)进行了杨树溃疡病菌接种试验,检测了树皮中5个抗病性相关基因的表达,对转多基因库安托杨溃疡病抗性进行了综合评价,为其推广应用奠定了基础,也为其它林木抗病分子育种提供参考。
HTML
-
植物材料为3个转多基因库安托杨株系(D5-19、D5-20和D5-21)及未转基因的对照株系(D5-0)。于2016年3月,选择越冬后植株高度在2 m左右、地径2.5 cm左右的1年生植株,从植株基部往上截取1.5 m的主干,将新截取的主干基部插入自来水中进行水培,培养条件为全自动控温日光温室,每天更换自来水,60 d后进行溃疡病病原菌接种。
接种菌株为葡萄座腔菌(Botryosphaeria dothidea)CZA菌株(保存于中国林科院林业新技术研究所)。菌株活化培养后,接种于PDA培养基进行暗培养,在28℃条件下培养10 d,取直径0.9 cm菌饼用于杨树主干接种。
-
距截取处30 cm高处开始,每隔约10 cm在枝干上均匀打孔,深度至露出木质部,打孔直径为0.9 cm,每个枝条打孔1012个。在打孔处接种杨树溃疡病CZA菌株菌饼,接种后用Parafilm密封接种处,同时在主干顶部封蜡以防止枝条失水。以打孔但未接种菌株的枝条(CK)作为对照,接种菌株枝条作为处理,对照和处理均设置5株作为生物学重复,试验重复3次。在接种后5 d后取接种孔间的健康树皮,液氮冷冻后置于超低温冰箱保存,用于提取总RNA。接种处理后第60天观察打孔处愈伤组织形成情况,并测量纵向溃疡斑大小。
-
参考黄逢龙等对杨树溃疡病病情指数的计算方法[2],结合本研究中苗木的发病状况,依据形成病斑的纵向长度,将病斑划分为5个不同的级别(表 1)。统计不同株系数据,计算杨树溃疡病病情指数。计算公式为:病情指数=[∑(各级病斑数×各级代表值)/(总接种点数×5)]×100。数据分析采用SPSS 19.0软件。
病级
Cankerrank代表值
Representative value分类标准
Standard of classificationⅠ 0 形成愈伤组织或不形成病斑 Ⅱ 1 病斑≤1.0 cm Ⅲ 2 1.0 cm < 病斑≤2.0 cm Ⅳ 3 2.0 cm < 病斑≤3.0 cm Ⅴ 4 3.0 cm < 病斑≤4.0 cm Ⅵ 5 4.0 cm < 病斑 Table 1. Classification standard of poplar canker disease
-
采用Qiangen RNeasy Plant Mini Kit提取接种点间健康树皮组织的总RNA。以Promega Super-Script TM Ⅲ第一链合成试剂盒反转录合成cDNA,并稀释10倍后用于RT-qPCR分析。使用Takara SYBR Premix Ex Taq试剂盒配制RT-qPCR反应体系,其中,cDNA模板体积为1.0 μL,其他成分为试剂盒推荐浓度。在Roche LightCycle 480Ⅱ型荧光定量PCR仪上对目标基因进行扩增,每个样品设置4个反应重复。选取杨树UBQ-like作为内参基因,抗病基因信息见表 2。PCR扩增程序为:预变性95℃,5 min;变性95℃,10 s,退火60℃,10 s,延伸72℃,10 s,45个循环。抗性基因的相对表达水平采用2-△△CT法进行计算[15]。
基因名称Gene name 引物序列Primer sequence 产物大小Product size/bp 几丁质酶基因
Chitinase gene5’-CCTCATGCCACAATGTCATC-3’
5’-CGATCCTCCACCTGTGATTT-3’148 链蛋白A基因
Chain A gene5’-TGGGTCTTCACGGTTTTCTC-3’
5’-ACATAGACCTCCCGGACACA-3’105 肉桂醇脱氢酶基因
Cinnamyl-alcohol dehydrogenase gene5’-CGTCAAGGTGACGGTGATTA-3’
5’-TCACGACTGACCAAAAACGA-3’90 病程相关蛋白基因
Pathogenesis-related protein gene5’-GCGATTGCAACCTTATCCAT-3’
5’-GCCTTCTCATCAACCCACAT-3’109 类甜蛋白基因
Thaumatin-like gene5’-GTATTGGTGGTGGTGCTCCT-3’
5’-AGCCAACATTGTAGCCATCC-3’111 内参基因
UBQ-Like5’-TGAGGCTTAGGGGAGGAACT-3’
5’-TGTAGTCGCGAGCTGTCTTG-3’195 Table 2. List of amplified genes and primer sequence
1.1. 实验材料
1.2. 方法
1.3. 病情指数计算
1.4. 树皮总RNA的提取、cDNA合成及RT-qPCR反应
-
接种溃疡病菌株60 d后,除去接种点的Parafilm,测量各株系枝条上的病斑纵向长度(图 1),将病斑数据换算为各病级和各病级代表值后,根据1.3中的病情指数公式计算各株系的病情指数,采用SPSS 19.0软件进行显著性差异检测。

Figure 1. The canker spots in bark of control and transgenic clones after B. dothidea inoculation for 60 days
实验结果(图 1)显示:非转基因对照株系D5-0的病情指数为27.59±1.36,转基因株系D5-19、D5-20和D5-21的病情指数分别为22.75±1.37、22.19±1.53和20.12±0.21。Fisher’s LSD检验结果(表 3)表明:对照株系病情指数极显著高于3个转基因株系,3个转基因株系中,D5-19与D5-20的病情指数间差异不显著,而D5-21的病情指数显著低于D5-19。抗病性数据分析结果表明:3个转基因库安托杨株系的溃疡病抗性较对照株系显著增强,同时,转基因株系间抗病性并不相同,D5-21株系对溃疡病具有更强的抗性。
株系
Clone病情指数
Disease index平均病情指数
Average disease
index显著差异性
Significant differenceT01 T02 T03 5% 1% D5-0 28.00 28.70 26.07 27.59±1.36 a A D5-19 22.41 24.26 21.59 22.75±1.37 b B D5-20 20.62 23.69 22.26 22.19±1.53 bc B D5-21 20.37 20.00 20.00 20.12±0.21 c B Table 3. Significant analysis of the canker disease index of transgenic poplar clones and the control
-
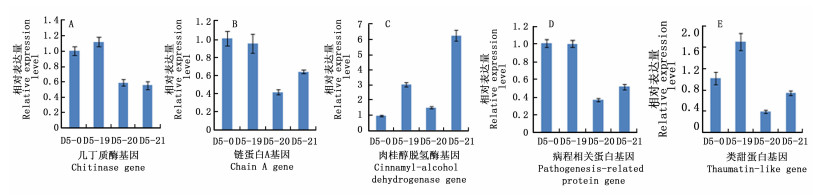
本研究对溃疡病病原菌接种5 d后,对照及转基因株系树皮中5个抗病相关基因的表达特征进行了相对定量分析。RT-qPCR结果显示:在溃疡病菌的胁迫后,不同抗病相关基因对溃疡病菌的响应差异较大(图 2)。

Figure 2. Expression pattern of 5 disease resistant genes in bark of control and transgenic clones after B. dothidea inoculation for 5 days
接种溃疡病菌5 d后,转基因株系及对照中几丁质酶基因(图 2A)、链蛋白A基因(图 2B)及病程相关蛋白基因(图 2D)的表达情况相似,即D5-19树皮中这3个抗病基因的表达量与对照相当,而在其他2个转基因株系树皮中这3个基因的表达量均低于对照。肉桂醇脱氢酶基因(图 2C)在3个转基因株系树皮中表达量均高于对照,其中,D5-20株系中的表达量为对照中的1.5倍左右,D5-19中的表达量为对照的3倍左右,D5-21中表达量为对照的6倍左右。类甜蛋白基因(图 2E)在D5-19树皮中的表达量高于对照,但在其他2个转基因株系中表达量均低于对照。综合分析接种溃疡病菌5 d后对照及转基因各株系树皮中5个抗病相关基因相对表达量发现,溃疡病侵染后,只有肉桂醇脱氢酶基因在3个转基因株系树皮中表达量均比对照有较大幅度的上升,并在D5-21中表达量最高;而类甜蛋白基因只在转基因株系D5-19中表达量高于对照。因此,转基因株系抗病性的提高与类甜蛋白基因及肉桂醇脱氢酶基因在转基因株系中的高表达密切相关。
2.1. 转基因库安托杨病情指数分析
2.2. 溃疡病胁迫下抗性相关基因表达量分析
-
近年来,随着分子生物学、分子遗传学和植物病理学研究的深入,发现植物抗病机制非常复杂[16-17]。当病原物侵染植物时,植物抗病反应网络开始响应,调节下游防卫基因表达。乙烯应答因子(ERF)是植物抗病信号响应的关键因子之一,ERF通过与下游基因启动子中顺式作用元件结合,调控下游多个防御性基因的表达,从而使植物产生抗病性[18]。近年来,已从拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、番茄(Solanum lycopersicum)、棉花(Gossypium spp.)、中间偃麦草(Thinopyrum intermedium)等多种植物中分离得到ERF转录因子基因,并证明ERF在植物抗病防御反应中起着重要的调控作用[19-21]。在拟南芥中,过表达ERF 1基因的植株对灰霉病[22]和枯萎病抗性明显提高[23]。Zuo等将海岛棉受黄萎病菌诱导的GbERF 1/2导入烟草后,发现ERF下游抗病相关基因表达量增加,转基因烟草对真菌病害的抵抗力明显增强[24]。另外,水稻稻瘟病菌(Magnaporthe grisea)能诱导水稻OsBIERF 1、OsBIERF 3和OsBIERF 4等ERF基因的表达[25];将OsBIERF 3基因导入烟草后,发现过表达OsBIERF 3基因的烟草植株对番茄花叶病毒和由烟草野火病菌(Pseudomonas syringae)引起的野火病抗性明显增强[26]。将小麦近缘种中间偃麦草的TiERF 1基因导入小麦品种‘扬麦12’中,发现TiERF 1基因能够通过激活病程相关基因几丁质酶和β-1,3-葡聚糖酶基因表达,进而提高转基因小麦对纹枯病抗性[27]。
本研究对人工接种溃疡病菌的转野生番茄ERF基因的库安托杨3个株系及对照进行了观测。通过病情指数的分析,发现转基因库安托杨株系抗病性均显著强于非转基因受体株系,说明转基因株系由于转入了与抗病性相关的JERFs基因,提高了库安托杨对杨树溃疡病的抗性。然而,转基因株系间抗病性并不相同,D5-21株系较其它2个株系对溃疡病具有更强的抗性。转相同基因的不同转基因植株在目标性状上的差异是在转基因研究中普遍存在的现象,其原因尚不明确[28-30]。
本研究对溃疡病菌胁迫下转基因库安托杨及对照中5个抗病基因表达情况进行了实时定量检测,结果发现,5个基因在溃疡病胁迫下,表现出复杂的表达模式。转基因株系中,只有2个抗病基因(肉桂醇脱氢酶基因和类甜蛋白基因)在全部或部分转基因株系表达量显著高于对照,而其他3个基因,在D5-19中与对照相当,而在另外2个转基因株系中,表达量都显著低于对照;另外,表达量升高的基因在各转基因株系间的表达量也有较大差异,在抗病性最强的D5-21中,肉桂醇脱氢酶基因表达量均较对照大幅上升。笔者认为,造成这种情况的原因可能是由于杨树对溃疡病胁迫的响应是一个涉及到多个基因、多种蛋白、多类转录因子的复杂调控过程[31],仅通过定量实验中选取的5个基因不足以发现在胁迫响应过程中的规律性变化。因此,后续研究应尝试通过转录组等高通量测序手段来揭示转基因杨树抗病性增强的分子调控网络。
作为植物抗病信号响应的转录因子,ERF基因的转入能够启动下游抗病相关基因的表达, 因此,可能赋予转基因植物对多种病害的抗性,如过表达MtERF 1-1基因的苜蓿植株,不仅提高了对立枯病(Rhizoctonia solani)的抗性,而且也提高了由苜蓿疫霉(Phytophthora medicaginis)引起的倒伏病抗性[32]。本研究仅检测了转多基因库安托杨株系对溃疡病菌的抗,转多基因库安托杨对其它杨树病害的抗性还有待于进一步研究。
-
通过对3个转多基因库安托杨株系的溃疡病抗病性检测及抗病相关基因的表达分析,发现在溃疡病菌胁迫下,转多基因库安托杨株系的溃疡病抗性与非转基因对照相比均有显著提高,且转基因株系间差异显著;同时,转基因株系树皮组织中不同抗病基因的表达模式也有很大差异,说明溃疡病菌胁迫下转多基因库安托杨抗病调控的复杂性,其分子调控机制还有待深入研究。本研究为通过基因工程手段培育抗病林木新树种提供有价值的参考。




 DownLoad:
DownLoad: