-
毛竹(Phyllostachys edulis (Carrière) J. Houz.)隶属禾本科刚竹属单轴散生竹,在竹类植物中具有用途最广泛、经营周期较短且经济效益较高的特点,已成为我国南方农林产业结构调整、林业增效、林农增收的主要经济林种[1]。我国现有毛竹林面积380多万公顷,占全国竹林面积的70%,占全世界毛竹面积的80%[1-2]。自毛竹枯梢病在浙江黄岩首次发现后,相继在南方各省份爆发成灾,造成严重经济损失,极大程度地制约了我国毛竹资源培育和竹产业的发展。该病害病原菌为竹喙球菌(Ceratosphaeria phyllostachydis),主要危害当年生新竹,造成枯株状态,已被列为国家森林植物检疫对象[3-5]。磷是植物生长过程中仅次于氮的第二大限制营养元素,约占植物干质量的0.2%[6],在生态系统能量代谢、核酸和蛋白质合成以及激酶调控等方面发挥着不可替代的作用[7]。由于土壤中仅有约0.1%的磷能直接被植物吸收利用,其余主要以难溶性磷酸盐存在[8]。因此,人们通过施用大量磷肥来满足植物对于磷素的需求,然而磷肥中的磷酸根离子极易与土壤中游离的Ca2+、Fe3+和Al3+等离子发生鳌合作用形成难溶性磷酸盐,导致施用磷肥中仅有5%~25%可被植物吸收利用,大量残余部分造成土壤磷素富集和土壤肥力丧失[9],引发了一系列的环境问题。这些亟待解决的问题使得提高毛竹抗病能力和竹林土壤磷素有效性成为一种迫切需要。
植物体内分布着丰富的有益内生细菌,它们不但可以通过分泌IAA、利用解磷和固氮等功能直接促进植物生长,还可通过分泌抗生素、水解酶等物质来帮助寄主植物提高抗病性,改善植物健康[10]。研究表明,多数内生细菌不仅存在于植物体内,也常存活于植物根际土壤环境中[11-12]。由于从土壤中筛选出的促生细菌在应用中发现,其在定殖过程中易受土著微生物的干扰导致定殖成功率不高、应用效果较实验结果欠佳[13]。因此,利用内生细菌进行植物病害防治和促生方面的应用已成为近年来的研究热点。然而目前关于毛竹内生细菌的相关研究仍较少,且对毛竹内生细菌抗病促生方面的研究也尚未见报道。故本试验对毛竹根系内生细菌进行分离,以竹喙球菌(C. phyllostachydis)作为指示菌进行拮抗菌株筛选,测定优良内生拮抗细菌的解磷能力和IAA等指标并进行种类鉴定。通过温室盆栽接种试验检验其对毛竹实生苗的促生长作用,以期为增强毛竹抗枯梢病能力,改善毛竹林地土壤养分情况,提高竹林生产力提供一条有效的生物途径,也为制备保护竹林健康及提高竹林生产力相关微生物菌剂提供优良的菌株资源。
HTML
-
毛竹枯稍病病原菌竹喙球菌(C. phyllostachydis)由中国林业微生物菌种保藏中心赠送,现保存于江西农业大学国家林业和草原局鄱阳湖流域森林生态系统保护与修复重点实验室。
-
牛肉膏蛋白胨固体培养基(NA):蛋白胨10 g,牛肉膏3 g,琼脂16 g,NaCl 5 g,水1 000 mL,pH 7.2。
牛肉膏蛋白胨液体培养基(NB):NA培养基不加琼脂。
马铃薯葡萄糖琼脂培养基(PDA):葡萄糖20 g,马铃薯200 g,琼脂18 g,水1 000 mL。
磷酸盐生长培养基(NBRIP):葡萄糖10 g,(NH4)2SO4 0.1 g,KCl 0.2 g,MgCl2 5 g,MgSO4·7H2O 0.25 g,Ca3(PO4)2 5 g,琼脂18 g,水1 000 mL,pH 7.0~7.2。
含溴酚蓝的磷酸盐生长培养基(NBRI-BPB):在NBRIP培养基中加溴酚蓝(0.025 g·L−1)。
-
样品分别采集于江西省官山林场、大井林场和大港林场毛竹林地,选取4~5年生毛竹,挖出根系,取部分细根装入无菌自封袋中,标明采样点、时间、编号等,置于4℃冰箱冷藏室备用。
-
毛竹根系用蒸馏水冲洗3遍,按下述步骤进行表面消毒:75% 酒精浸泡1 min,3.25%次氯酸钠浸泡3 min,75%酒精浸泡30 s,后用无菌水冲洗3遍,无菌滤纸吸干表面水分,进行研磨,收集研磨液[14]。用最后一次冲洗后的无菌水涂布NA平板,检验是否表面灭菌彻底。采用稀释平板法[15],将纯化的单菌落于NA斜面培养、储存、备用。
-
将培养5 d后的竹喙球菌菌饼放置在PDA平板中央,将分离所得的细菌采用划线法接种于PDA平板两侧,设置仅接病原菌的PDA平板为对照,各处理3个重复。置28℃恒温箱内培养4 d后测量抑菌带宽度并做标记。将抑菌效果较好的菌株PN1和PN6接种于NB培养基中。用150 mL摇瓶进行发酵液培养,每瓶装液量50 mL,于30℃(200 r·min−1)培养2 d。将培养后的发酵液离心取上清液经0.22 μm微孔滤膜过滤。混合5 mL无菌滤液与20 mL PDA培养基后倒平板;以等量无菌水代替无菌滤液为对照,接竹喙球菌菌块于平板中央培养4 d(28℃),各处理3个重复。测量并计算各处理生长抑菌率。计算公式如下:
-
采用比浊法测定菌株PN1和PN6的生长曲线,将菌株PN1和PN6在NB培养基中震荡培养,每隔6个小时取样,测定培养液的OD600值,并绘制生长曲线。
-
采用Pikovskaya等人的方法对菌株PN1和PN6进行解磷能力的定量测定。将菌株PN1和PN6在NB培养基震荡培养24 h,配制菌液浓度为1×108 cfu·mL−1后,将菌液按1%的接种量至50 mL NBRIP培养液中,以等体积未接菌NBRIP培养液为对照,置于30℃、180 r·min−1环境下摇培5 d,各处理5个重复。将发酵液于4℃、10 000 r·min−1条件下离心10 min,取各处理上清液采用钼锑抗比色法测其可溶性磷含量[16]。
-
菌株产IAA量采用Salkowski比色法测定。将IAA标样稀释至0,0.5,2.5,5.0,7.5,10,12.5,15,17.5,25,50 mg·L−1,将各浓度梯度的IAA与FeCl3比色液等比例混合(2 mL)在30℃下暗储藏30 min后,测其在波长530 nm条件下的吸光度,并绘制标准曲线。培养PN1和PN6菌液15 d,以未接菌的NB液体培养基为对照。菌悬浮液与对照离心10 min(10 000 r·min−1)后取上清液进行IAA含量的测定,IAA含量测定采用标准曲线制作方法[17]。
-
菌株PN1和PN6形态与生理生化分析参考《常见细菌系统鉴定手册》[18]和利用Biolog鉴定,Biolog系统鉴定方法具体步骤参照该系统的鉴定说明书。DNA提取采用CTAB法[19],采用1495 r和27 f引物,PCR产物送金斯瑞生物科技公司测序,测序得到的序列提交至NCBI数据中并进行BLAST比对分析,在NCBI数据库中下载相关基因序列,利用软件MEGA 5.0构建系统进化树[20]。
-
将菌株PN1和PN6活化后,用接种环挑取少量菌体接种于NB培养基中28℃,180 r·min−1振荡培养48 h。发酵液(4℃,5 000 ×g)离心5 min,无菌生理盐水润洗菌体3次并调节菌悬液(108 cfu·mL−1)制成液体菌剂。采用完全随机设计试验,培养基质按照土壤∶砂子∶蛭石=2∶1∶1的配比混合,设置对照(等量无菌生理盐水)、单独施用解磷菌PN1和PN6 3个处理。于当年4月份采用灌根的方式分别施用于毛竹根际土壤里,菌剂施用量为10 mL·株−1,各处理20 株。在栽培180 d 时测定毛竹苗高、地径。
-
利用Microsoft Excel 2013进行数据整理,采用SPSS和Origin分析软件做统计和制图,运用单因素方差分析抑菌活性、解磷量和分泌IAA量和促生试验数据(P<0.05)。图表中数据为平均值±标准误。
1.1. 试验材料
1.1.1. 试验菌株
1.1.2. 培养基种类
1.1.3. 样品采集
1.2. 方法
1.2.1. 毛竹根系内生细菌的分离
1.2.2. 内生拮抗细菌的筛选
1.2.3. 内生拮抗细菌PN1和PN6生长曲线的测定
1.2.4. 内生拮抗细菌PN1和PN6解磷能力的测定
1.2.5. 内生拮抗细菌PN1和PN6分泌IAA能力测定
1.2.6. 内生拮抗细菌PN1和PN6鉴定
1.2.7. 内生细菌PN1和PN6对毛竹促生作用
1.3. 数据处理
-
细菌分离数量结果显示,毛竹根系存在丰富的细菌资源,3个采样点共分离纯化得到118株细菌,各采样点均分离到约40株内生细菌(表1)。
采样地
Sampling site经度
Longitude维度
LatitudepH 海拔
Elevation/m内生细菌数量
Quantity of endophytic bacterial大港林场
Dagang Forest Farm114°56′31″ E 28°37′17″ N 5.16±0.09 a 381.0 43 官山林场
Guanshan Forest Farm114°34′47″ E 28°33′35″ N 4.37±0.07 b 548.0 37 大井林场
Dajing Forest Farm114°8′19″ E 26°34′8″ N 4.39±0.06 b 1 105.0 38 总计 Total — — — — 118 注:不同字母表示3个采样点间差异显著(P<0.05)。下同。
Note: Different letters indicated the significant differences among the three sampling sites at the level of P<0.05. The same is below.Table 1. The quantity of bacteria from Ph. edulis roots
-
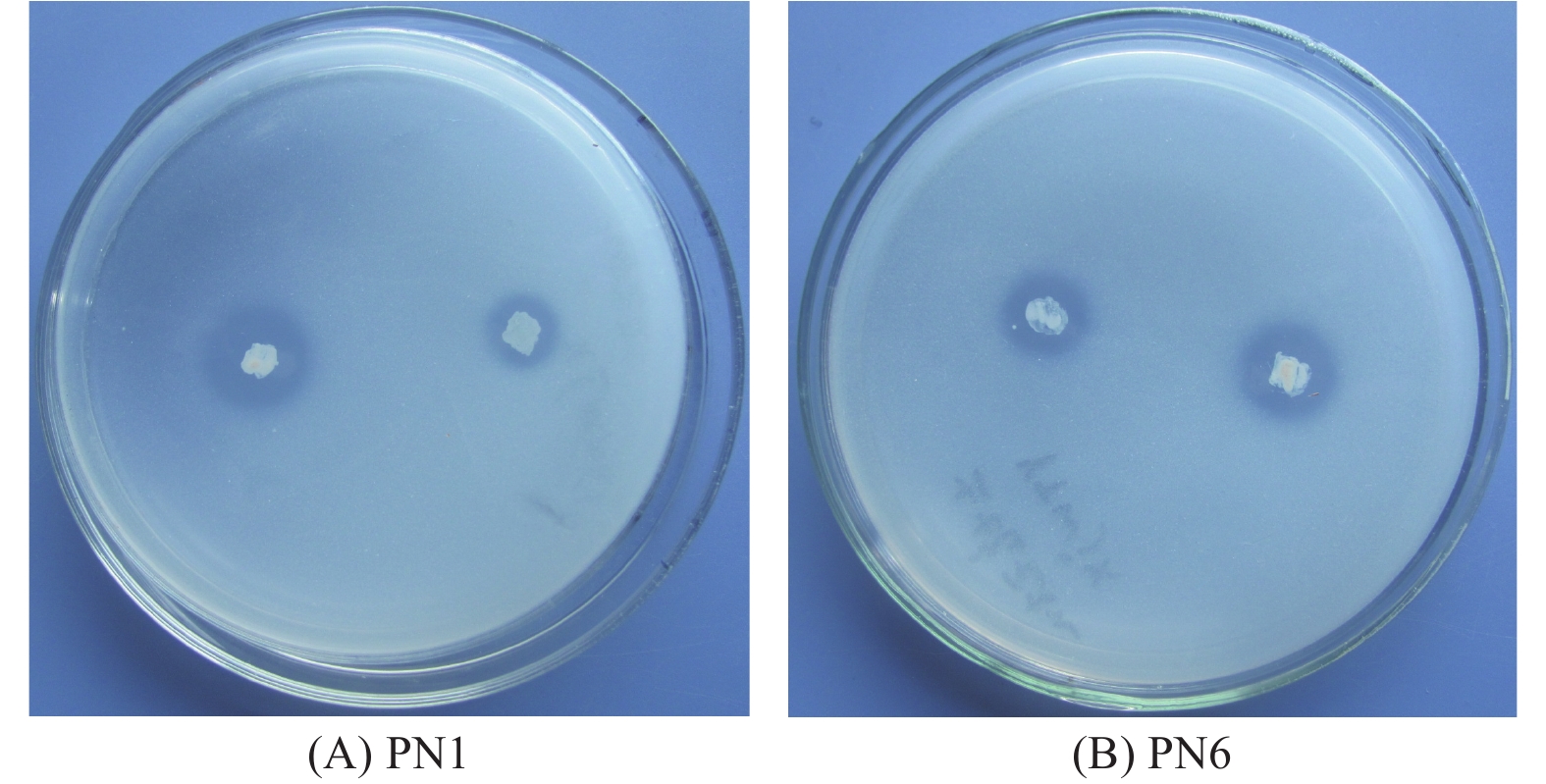
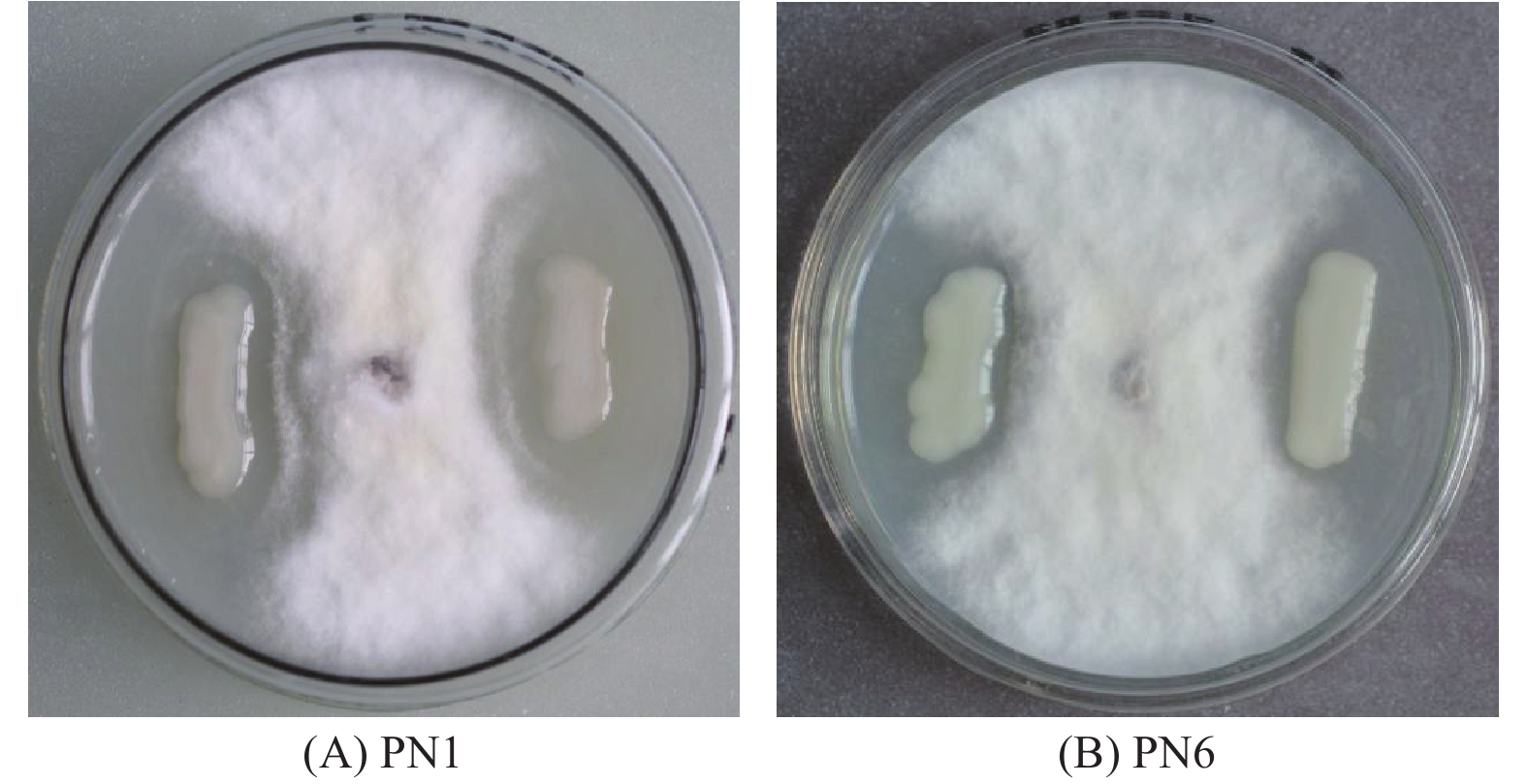
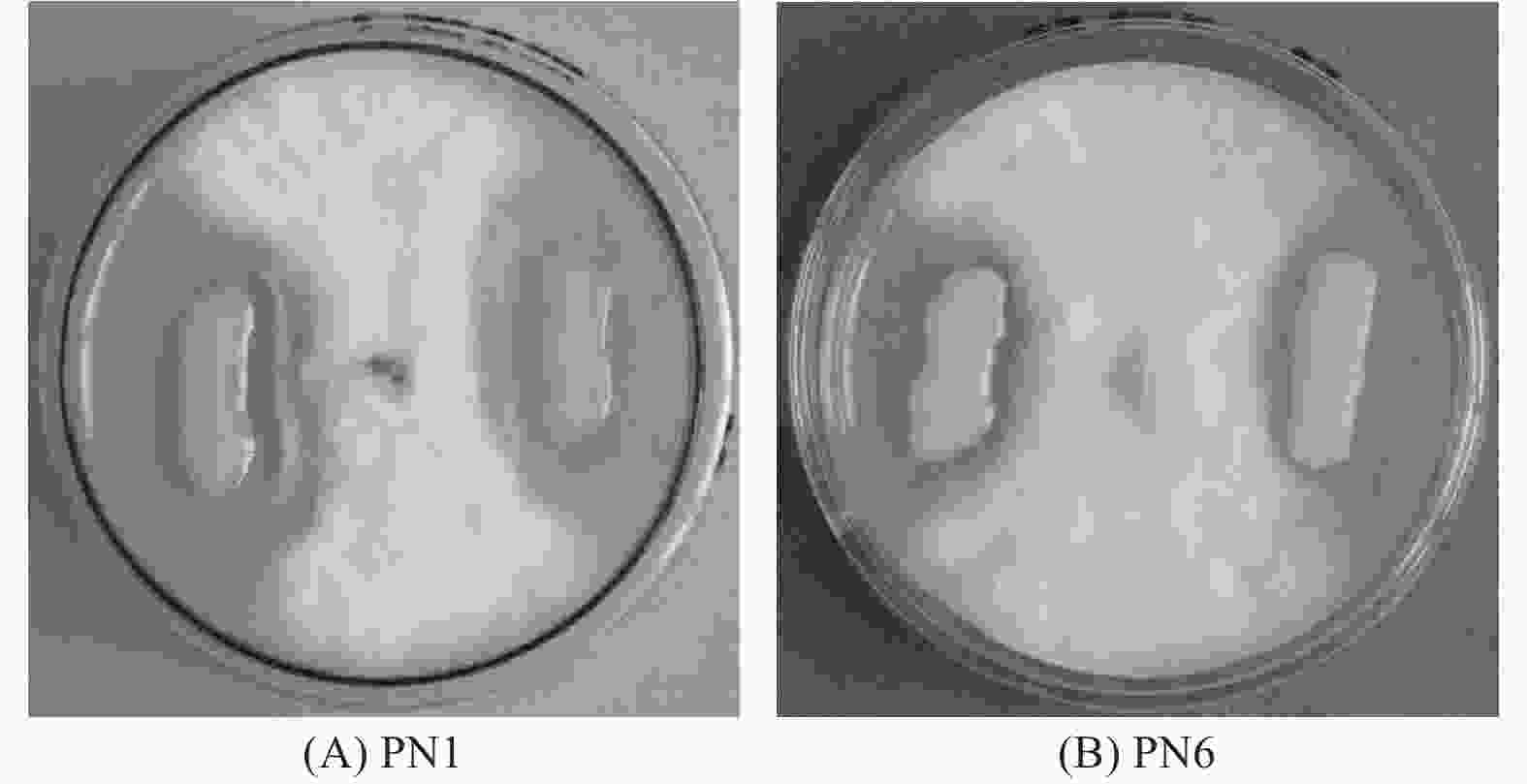
通过平板对峙试验,从毛竹根系中分离纯化的118株细菌中,对竹喙球菌有拮抗作用的菌株有16株,其中菌株PN1和PN6对病原菌竹喙球菌有较强抑菌活性(图1)。平板抑菌带宽度分别可达2.81 cm和2.13 cm(表2)。菌株PN1和PN6发酵液对竹喙球菌也具有较好的抑菌活性,抑菌效果分别可达58.82%和49.28%(表2)。
菌株
Strain发酵液的抑菌活性
Antibacterial activity of fermentation broth平板抑菌带宽度
Inhibition
zone diameter/cm菌落直径
Colony diameter/cm抑菌率
Inhibitory rate/%PN1 4.33±0.11 a 58.82 2.81 PN6 4.97±0.11 a 49.28 2.13 CK 9.8±0.06 b — — Table 2. The antagonistic function of antagonistic bacteria PN1 and PN6 to pathogenic fungi
-
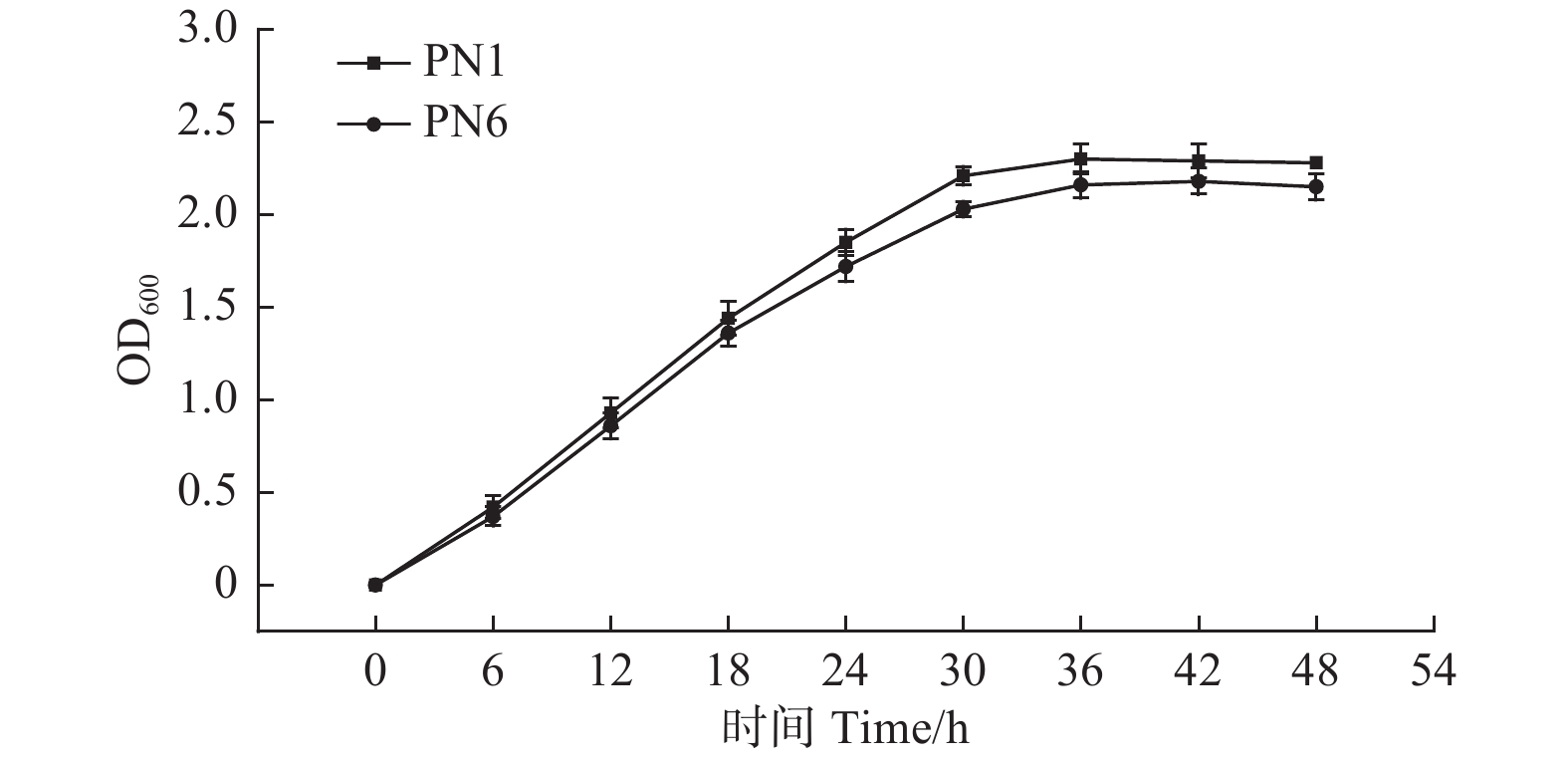
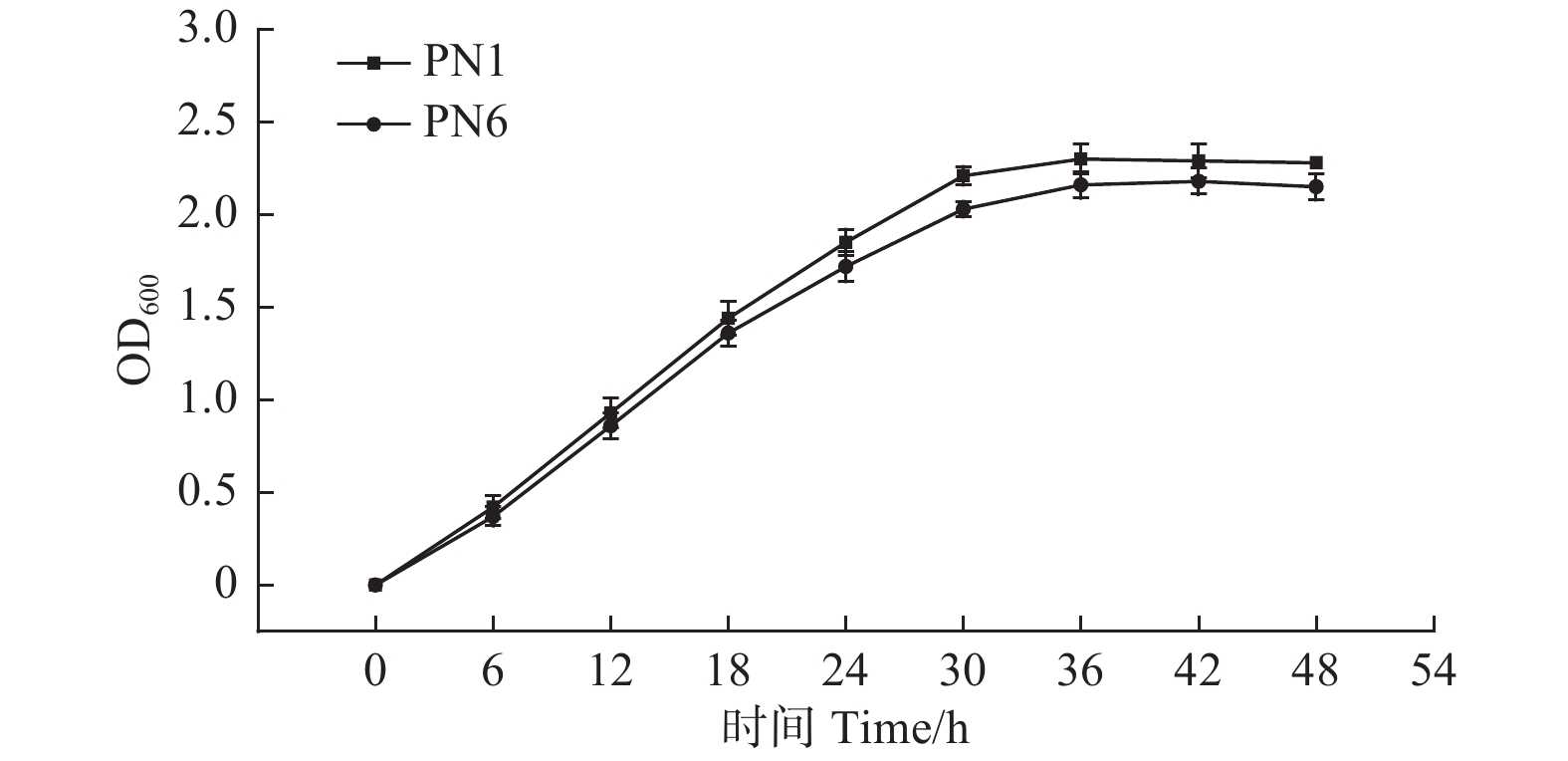
由图2可知,菌株PN1和PN6的菌液OD600值在0~30 h增长较快,菌株PN1在接种后约36 h内达到最大值2.30,而菌株PN6在接种42 h后达最大值2.18。之后2株细菌的OD600值的变化差异不明显,标志菌株生长进入稳定期(图2)。血球计数板计数结果显示,培养液中菌株PN1和PN6的菌体密度在培养36 h和42 h后分别增加了约125倍和116倍,且菌株PN1的生长能力高于菌株PN6。
-
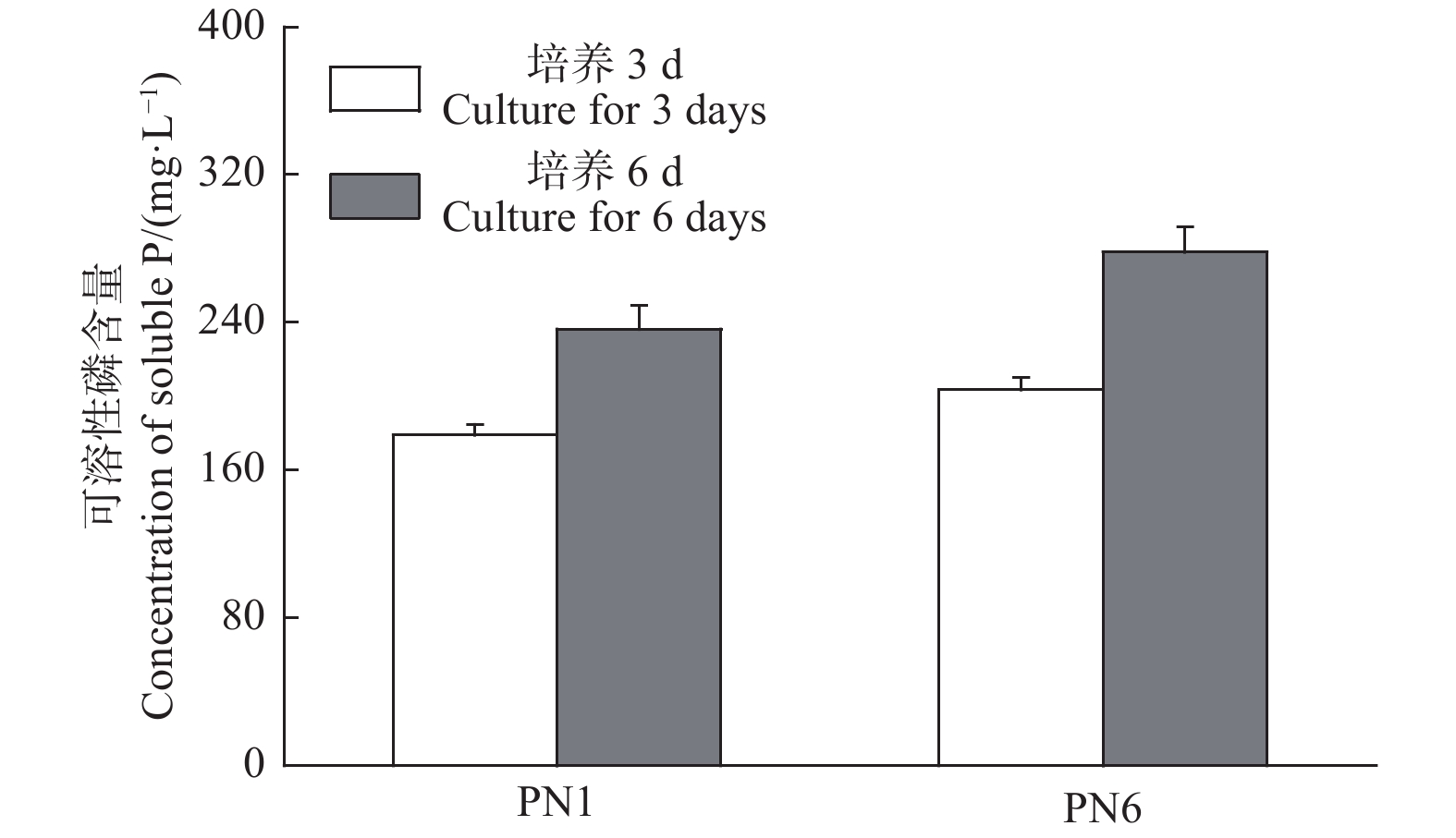
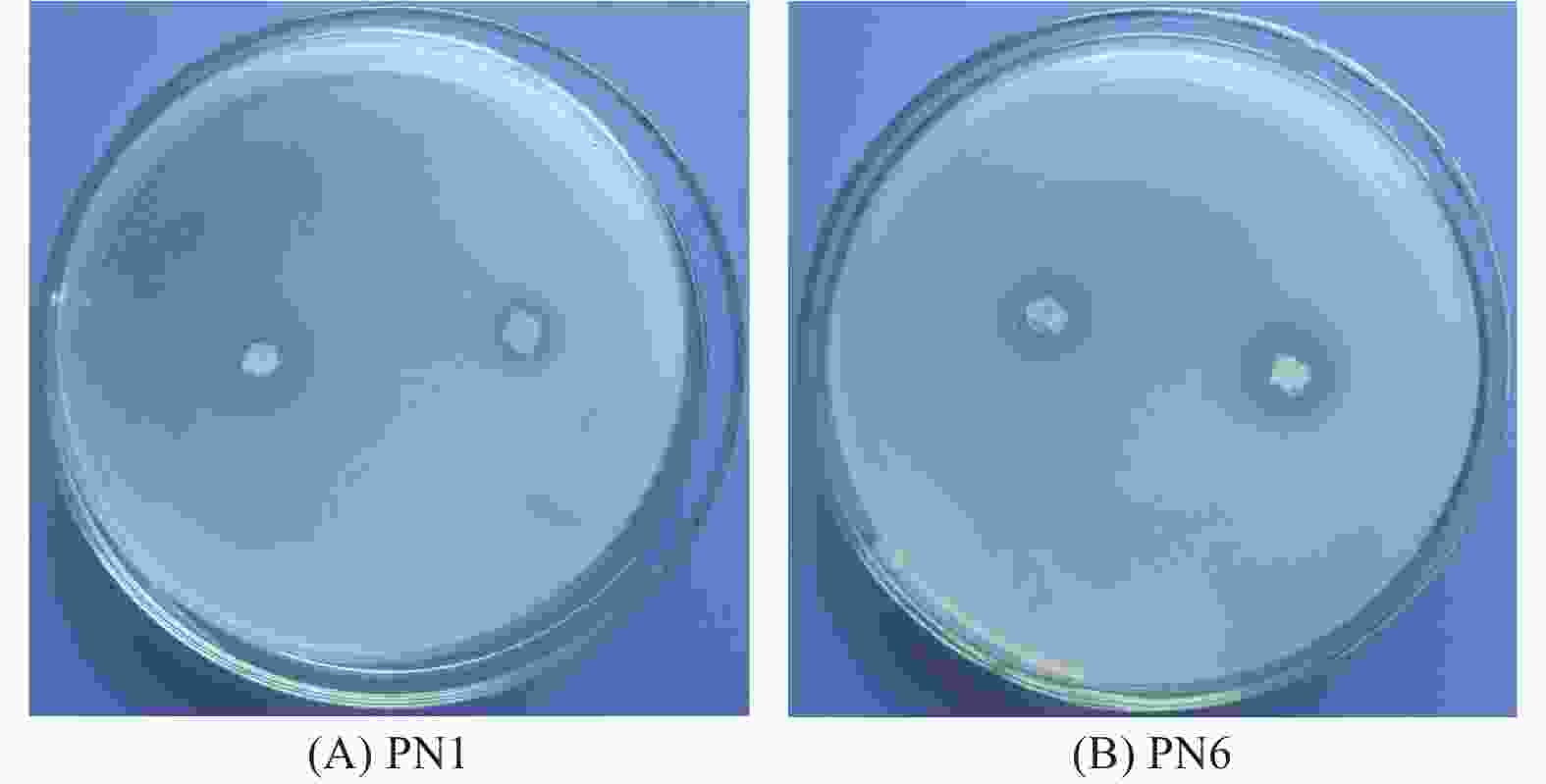
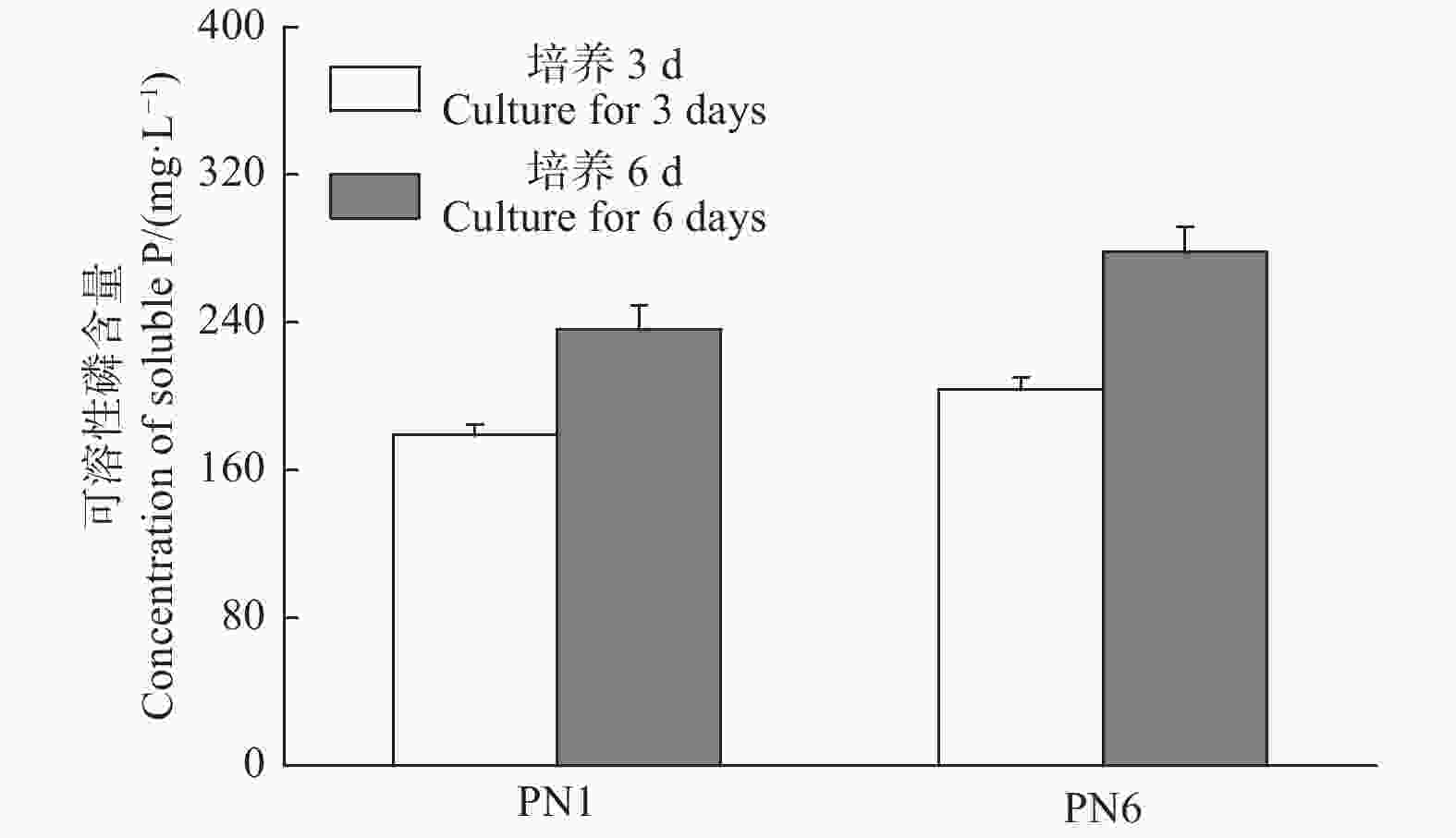
菌株PN1和PN6在NBRIP固体培养基均有解磷圈,具有较好的解磷能力(图3)。将菌株PN1和PN6分别接种于NBRIP-BPB液体培养基中振荡培养3 d和6 d后,测定菌株PN1和PN6对磷酸三钙的溶解能力。结果显示,这2株细菌在第3 d和6 d均具有较强的解磷能力,菌株PN1解磷量分别为179 mg·L−1和236.33 mg·L−1,菌株PN6解磷量分别为203.67 mg·L−1和278.21 mg·L−1(图4)。
-
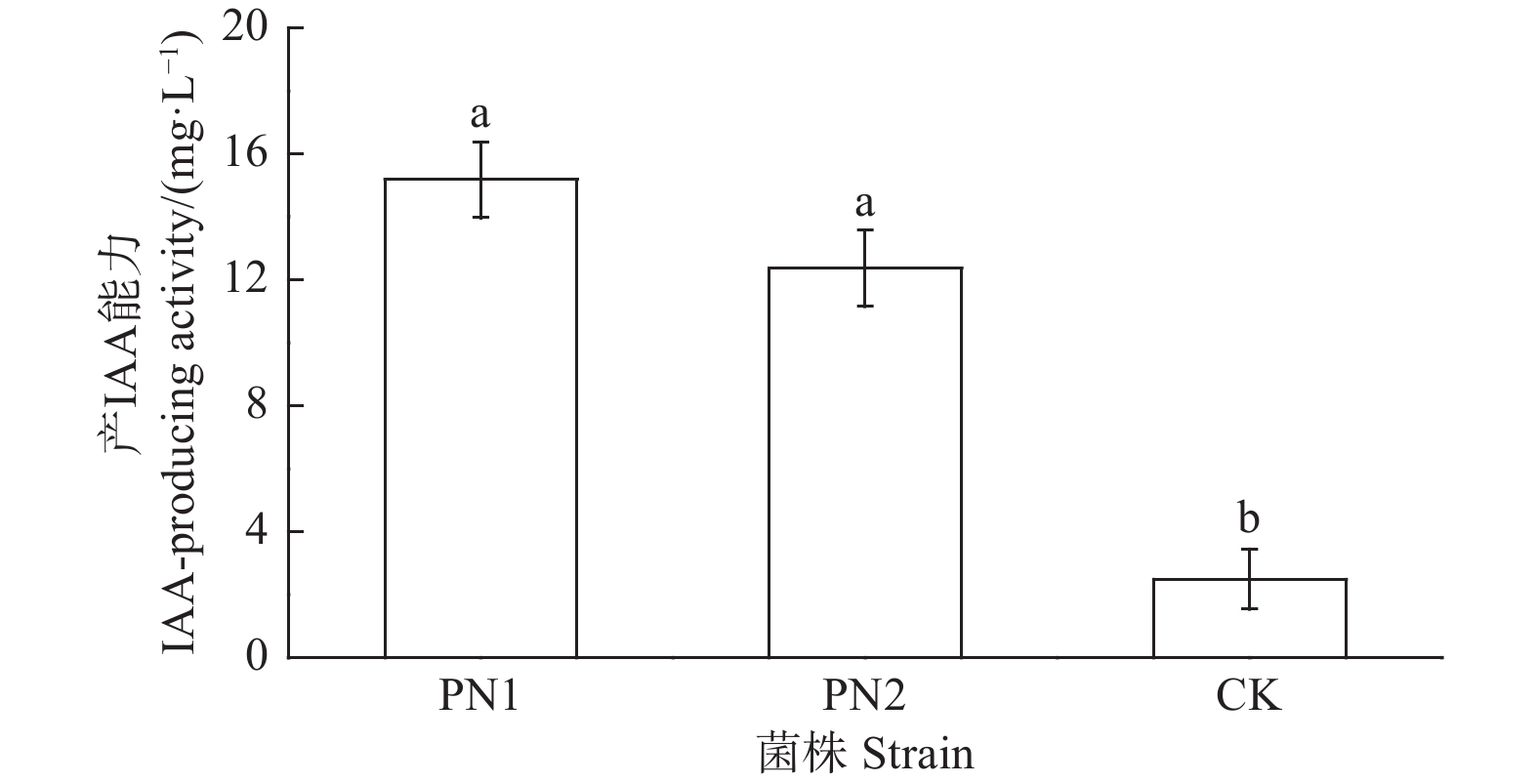
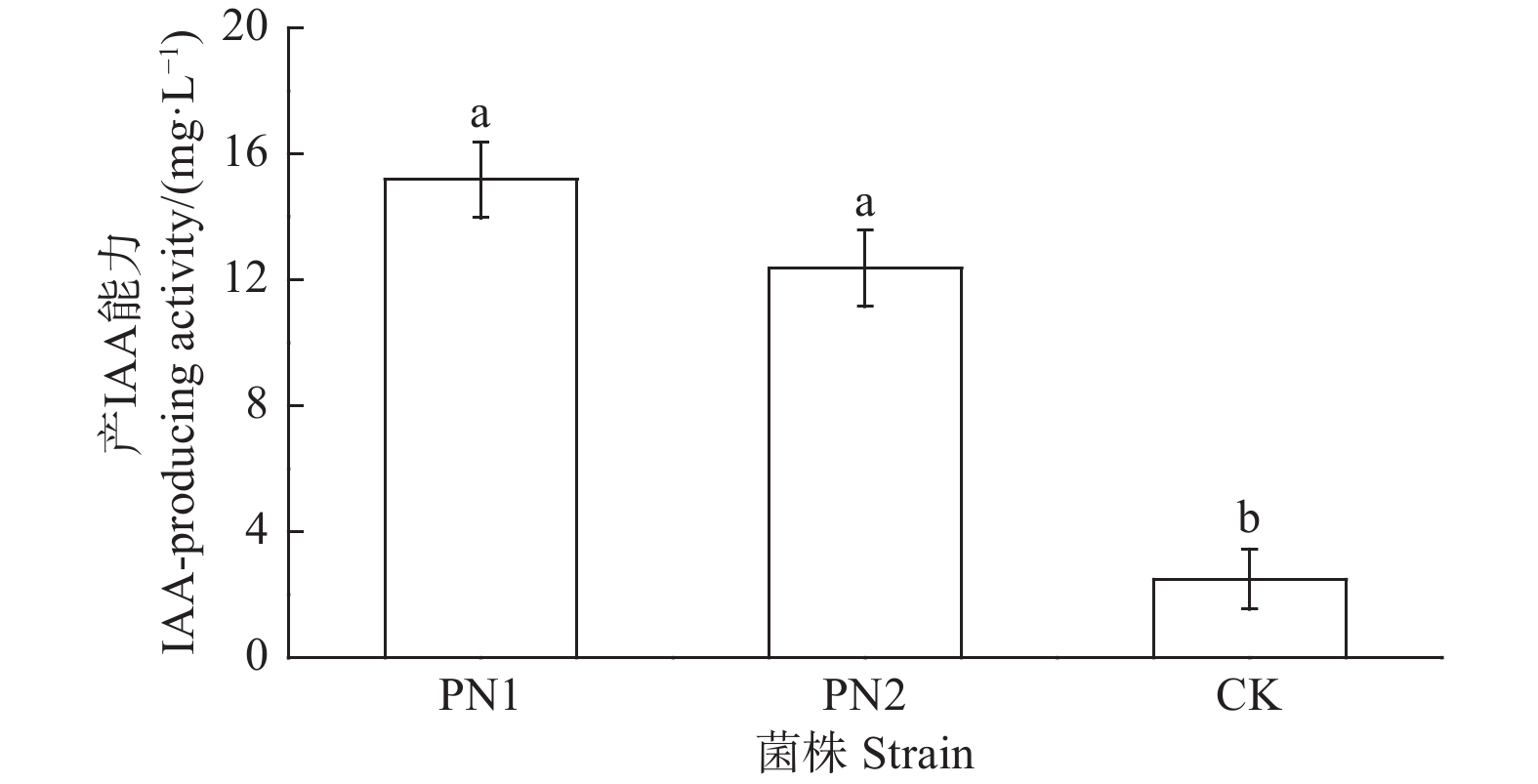
采用Salkowski比色法对菌株PN1和PN6定量测定,结果显示菌株PN1和PN6有着较好的分泌IAA能力,其IAA分泌量分别为15.17 mg·L−1和12.36 mg·L−1(图5)。
-
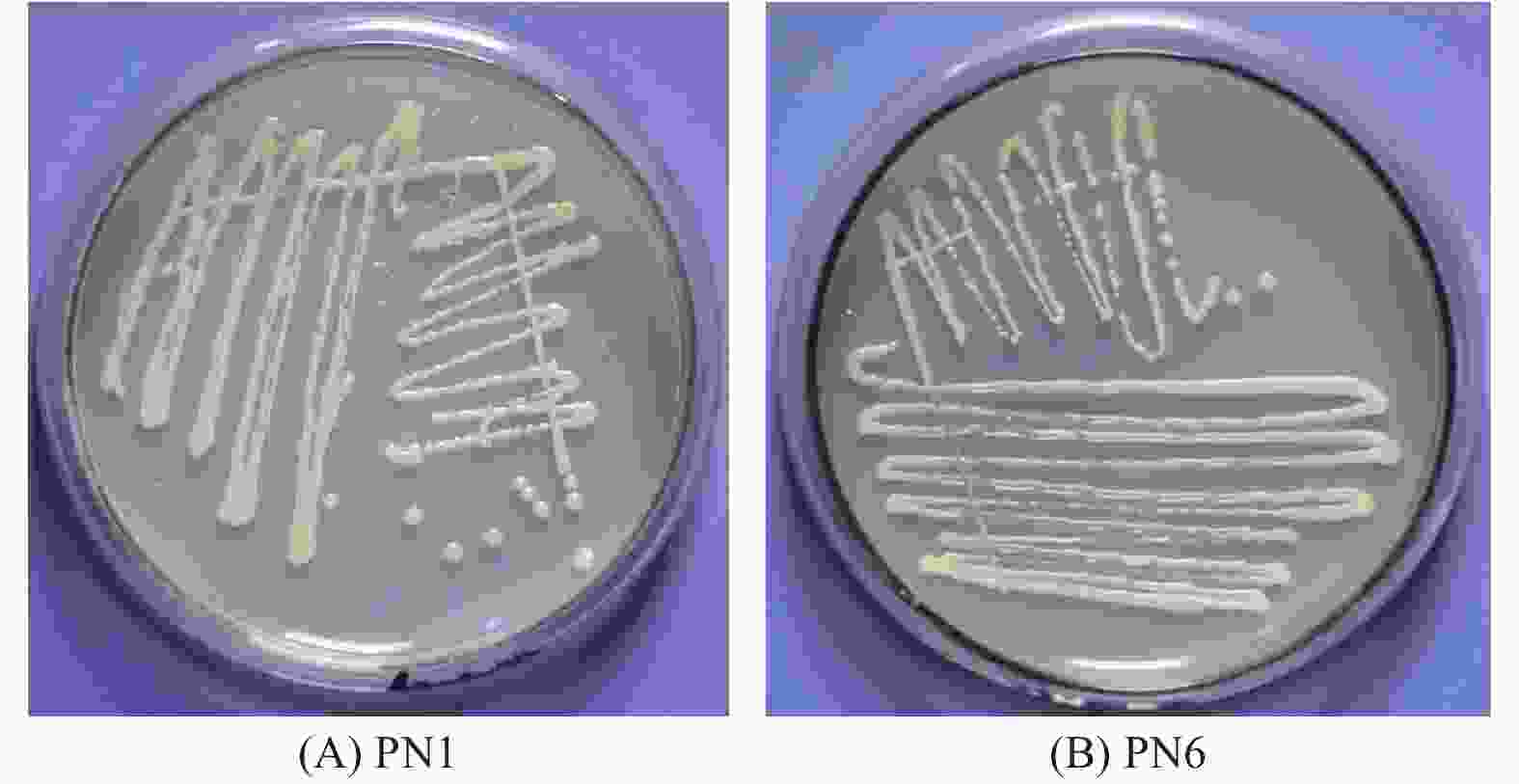
菌株PN1和PN6在NA培养基上培养48 h后,菌落均呈圆形,颜色呈淡乳白色,不透明(图6)。显微观察结果显示,2株细菌均为杆菌;生理生化特征分析显示,2株细菌均属于好氧性革兰氏阴性杆菌(表3)。
测定指标 Measurement index PN1 PN6 3%KOH溶解性实验3% KOH solubility test − + 接触酶实验 Contact enzyme test + + 氧化酶 Oxidase + − 明胶水解 Gelatin hydrolysis − + 淀粉水解实验 Starch hydrolysis test − + 柠檬酸盐利用实验 Citrate utilization test + + 吲哚试验 Indole test + − 甲基红试验 Methyl red test − + 硝酸盐还原实验 Nitrate reduction test − + 丙二酸盐利用实验 malonate utilization test + + 伏普实验 Voltam test − − 革兰氏染色 Gram’s dye G− G− 需氧性实验 Aerobic experiment 需氧
Aerobic需氧
Aerobic菌体形态 Bacterial morphology 杆状
Rhabditiform杆状
rhabditiform备注:“−”表示反应阴性;“+”表示反应阳性
Note: "-" indicates negative reaction; "+" indicates positive reaction.Table 3. Biochemical and physiological characteristics of strains PN1 and PN6
-
将分离纯化的PN1和PN6于Biolog鉴定板30℃恒温培养18~24 h后,置Biolog自动微生物鉴定仪上读数到相似值(SIM)均大于0.5,符合鉴定系统的要求。菌株PN1和PN6分别鉴定为Burkholderia lata和Enterobacter ludwigii。
-
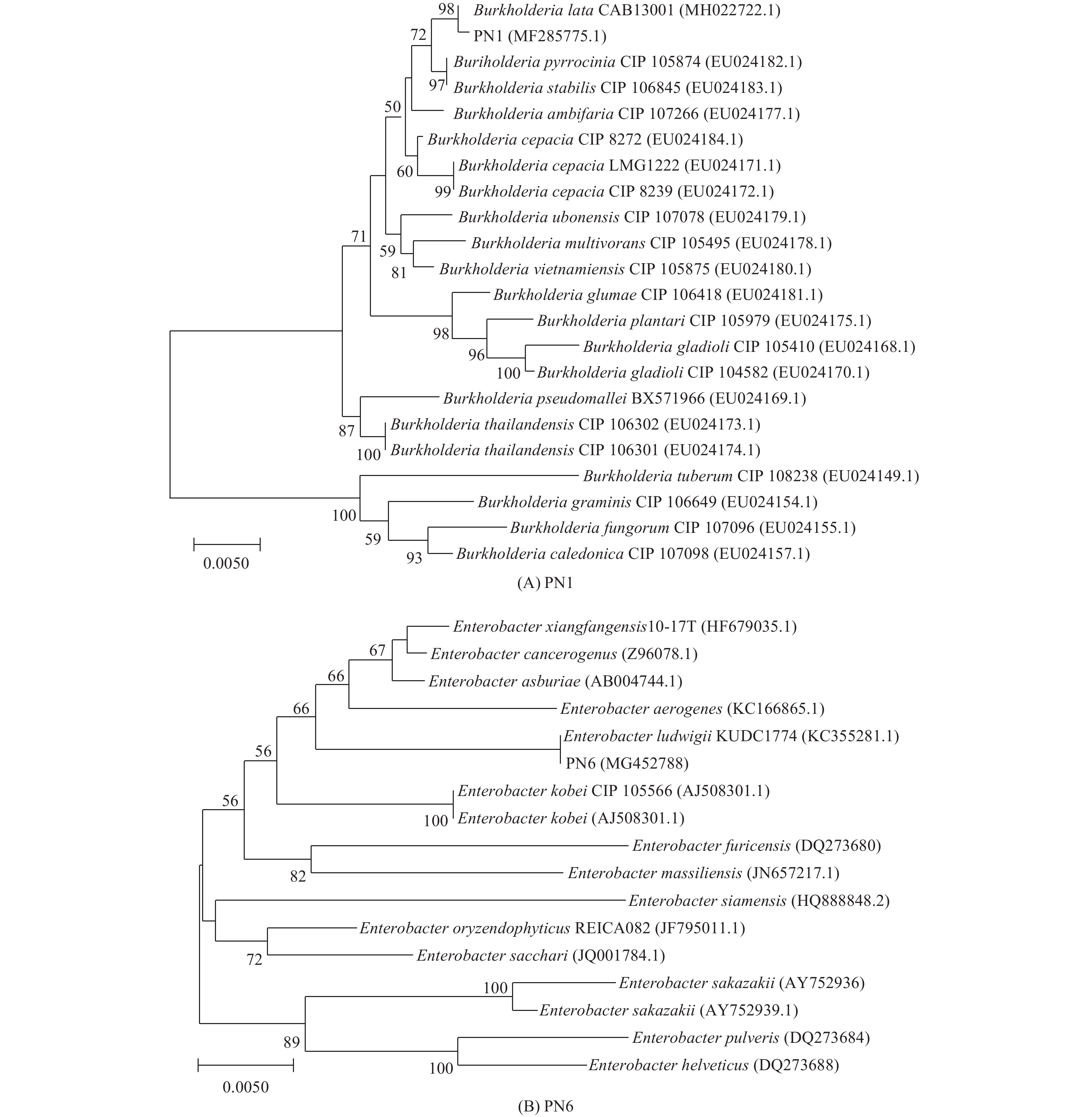
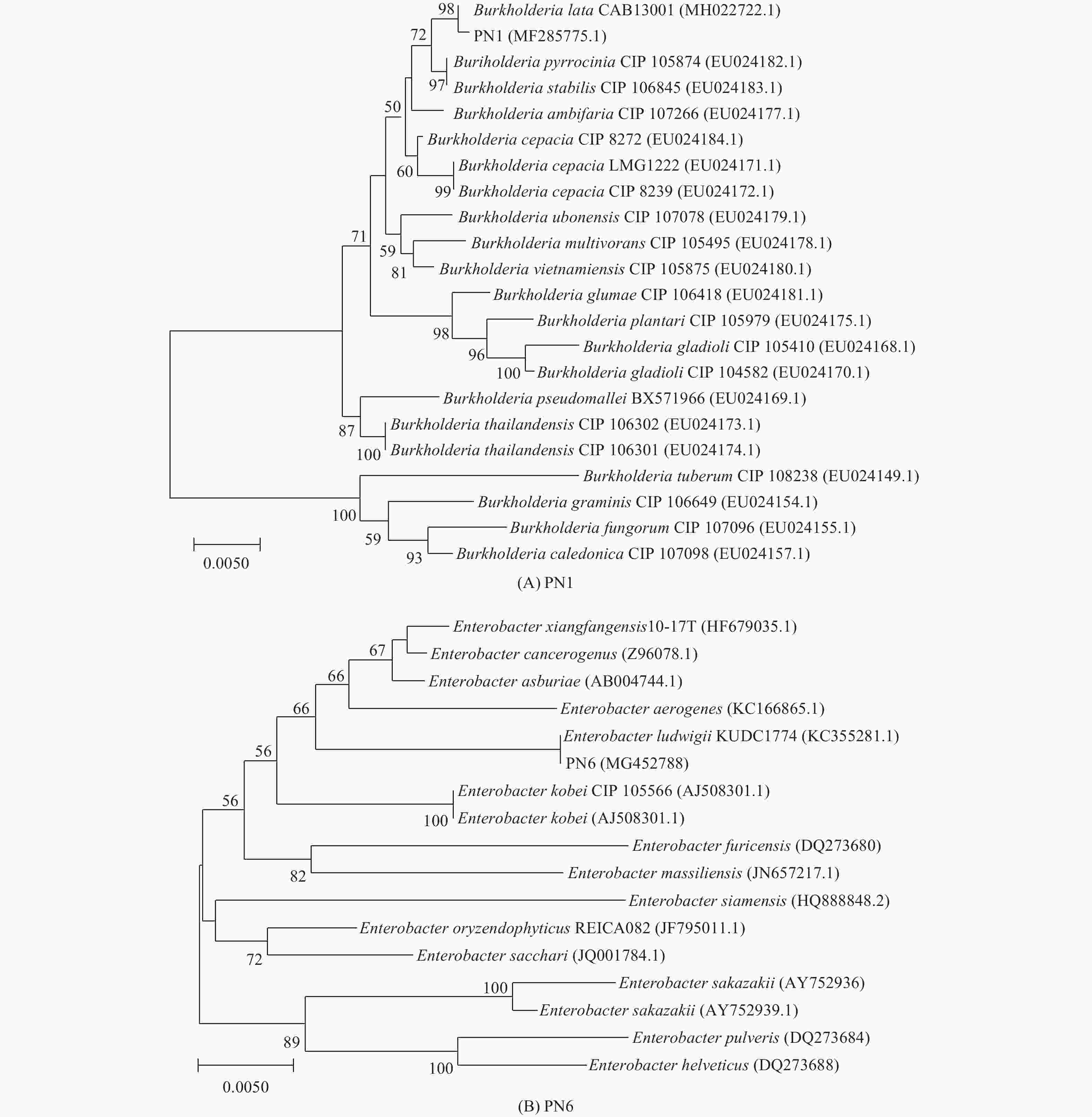
菌株PN1和PN6的16S rDNA基因序列已提交至GenBank中(登录号为MF285775和MG452788)。由图7的系统进化树可知,菌株PN1与Burkholderia lata聚类同一个分支,相似度达98%,菌株PN6与Enterobacter ludwigii聚类同一个分支,相似度达100%,说明具有较接近的亲缘关系,参照菌落形态特征和生理生化等指标,菌株PN1鉴定为B. lata,PN6鉴定为E. ludwigii。
-
施用菌剂180 d后菌株PN1和PN6均能促进毛竹实生苗的生长(图8)。施菌处理后毛竹的苗高和地径均显著高于对照,菌株PN1和PN6处理的地径增长率分别为39.51%和42.59%,苗高增长率分别为54.42%和62.51%(表4)。
处理Treatment 地径Ground diameter/cm 增长率Growth rate/% 苗高Seeding height/cm 增长率 Growth rate/% PN1 2.26±0.04 a 39.51 35.13±0.74 a 54.42 PN6 2.31±0.07 a 42.59 36.97±0.83 a 62.51 CK 1.62±0.2 b - 22.75±0.64 b - Table 4. The effect of adding strains PN1 and PN6 on the growth of Ph. edulis seedling (180 d)
2.1. 毛竹根系内生细菌的分离及纯化
2.2. 毛竹枯梢病病原菌内生拮抗细菌的筛选
2.3. 菌株PN1和PN6生长曲线比较
2.4. 内生拮抗细菌PN1和PN6解磷能力的测定
2.5. 内生拮抗细菌PN1和PN6产IAA能力
2.6. 菌株PN1和PN6的鉴定
2.6.1. 菌株PN1和PN6的形态、生理生化特性
2.6.2. 菌株PN1和PN6的Biolog鉴定
2.6.3. 菌株PN1和PN6的16S rDNA鉴定
2.7. 菌株PN1和PN6对毛竹实生苗的促生长作用
-
植物体内分布着多种内生真菌、细菌等有益微生物,这些微生物与植物在长期发育进化过程中形成了稳定的定殖环境,在维持植物健康方面发挥着重要作用[21]。目前利用内生细菌对植物病害进行生物防治方面的研究从广度到深度均取得了快速发展[22-23]。如Orawan等人从柑桔中分离出内生细菌芽孢杆菌对柑桔溃疡病有较好的拮抗作用[24];李亮亮从松树体内分离出3株细菌,经发酵液浸渍试验显示3株细菌对松材线虫有较高杀线活性[25];Wicaksono从猕猴桃分离出5株内生细菌,拮抗试验显示5株细菌对猕猴桃细菌性溃疡病病原菌丁香假单胞菌Pseudomonas syringae具有较强的拮抗作用,并发现其中3种内生细菌能通过接种试验成功定殖在猕猴桃体内,显著降低溃疡病的发生程度[26]。一系列研究结果表明,内生细菌在防治植物病害方面具有一定功效,且有较强的适生性,易在植物体内定殖、形成种群优势,不会对生态环境造成危害[11, 21]。因此,本试验筛选出菌株PN1和PN6可为今后生物防治毛竹枯梢病时优先考虑应用的菌株。迄今关于拮抗细菌抗病性的相关机制可能是其产生某些抗病原菌的低分子量化学物质,如木质素、胼胝质和多聚物等,也可能是通过诱导一些水解酶、氧化酶类以及相关蛋白的产生进行[27]。本试验筛选的菌株PN1和PN6分别为Burkholderia lata和Enterobacter ludwigii,以往研究显示,Burkholderia sp.对病原菌的抑制机制主要与其产生的木假丝菌素、洋葱菌素吩嗪、硝吡咯菌素和赛派素等相关代谢产物密切相关[17],Enterobacter sp.对病原菌的抑制机制主要是通过产生几丁质分解酶来抑制病原菌活性[28]。本研究中这2株细菌产生何种代谢产物来抑制竹喙球菌尚不清楚,具体拮抗机制还有待研究,以便在今后防治毛竹枯梢病时发挥其最大潜力。
近年来,内生细菌除了具有防治植物病害的能力外,其它生物学功能也屡见报道[10, 29]。如姚玉玲从高寒草地矮生嵩草中分离1株内生细菌263AG5,发现该菌株对马铃薯炭疽病病原菌有较好的抑制作用,对磷酸三钙具有较好的溶解作用,此外还具有固氮和产IAA功能[30];Daniel筛选出2株内生细菌E25和CR71,能释放挥发性有机化合物,对植物灰霉病有显著的拮抗作用,温室试验显示这2株细菌能显著提高番茄叶绿素含量和生物量[31];Zhao从河南大豆根瘤中分离276株内生细菌,通过对大豆疫霉菌的拮抗试验筛选出6株抑菌活性较强的细菌,并发现这些菌株具有产铁载体、IAA及固氮功能[32]。与已报的功能菌株相比,本研究中筛选出的菌株PN1和PN6具有较好的分泌IAA能力和抑菌功能,且对磷酸三钙具有较好的溶解作用,其产IAA和解磷能力可比其他菌株强至2~3倍[26,30,32],盆栽接种试验也显示这2株细菌对毛竹实生苗具有显著的促生长作用,该结果显示了这2株细菌具有多功能特性,具有开发成为抗病促生相关生物菌剂的潜力。以往部分研究证实内生细菌可通过抑制病原菌的侵染、提高寄主对逆境的抗性和通过产生植物激素、提高植物对有效养分的吸收等方式来促进植物生长[33]。我国毛竹主要分布于江西、浙江等亚热带丘陵红壤地区[34],这些地区土壤由于水土流失严重导致氮、磷、钾等养分供应不足,极大地限制了竹林的持续经营和利用[35]。如最近研究表明磷已成为毛竹产量提升的主要制约因子[36]。因此,筛选出具有多功能生物学特性的细菌对于防治毛竹枯梢病及提高竹林土壤养分含量是一条有效的生物途径[34]。而本试验所筛选的菌株PN1和PN6既能降低在定殖于毛竹体内时的生防效应,又能较小受到来自土著微生物的干扰,为毛竹生长过程提供有效养分,进而提高竹林生产力。同时,本研究的结果也扩充了促生细菌种质资源,为改善毛竹养分有效性提供了新的生物途径,也间接证实内生细菌也是优良促生菌株的重要资源库[37]。
-
本研究从毛竹根系中分离到16株对毛竹枯梢病病原菌竹喙球菌(C. phyllostachydis)具有拮抗作用的内生细菌,其中菌株PN1和PN6的抑菌活性最显著,这2株细菌也具有较好的解磷能力和分泌IAA能力。菌株PN1和PN6分别被鉴定为Burkholderia lata和Enterobacter ludwigii。盆栽结果显示2株细菌均能显著促进毛竹实生苗生长。以上结果表明这2株细菌具有多功能特性,具有开发成为抗病促生相关生物菌剂的潜力。




 DownLoad:
DownLoad: